Figure 1.
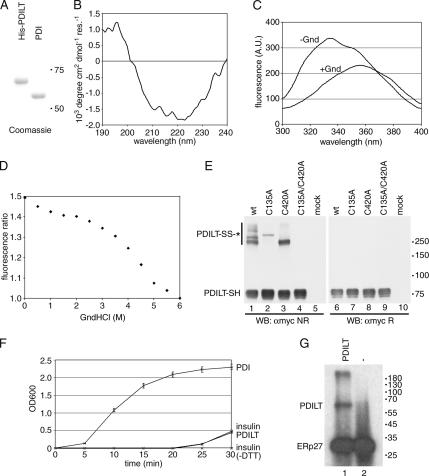
PDILT has chaperone-like properties. (A) Purified human His-tagged PDILT and purified rat PDI were analyzed by SDS-PAGE and Coomassie staining. (B) Far-UV circular dichroism spectrum of purified recombinant human PDILT. (C) Fluorescence spectra of purified human His-tagged PDILT under native (−Gnd) and denaturing conditions (+Gnd). (D) Guanidine hydrochloride denaturing curve of purified human His-tagged PDILT. Shown is the ratio of the average fluorescence intensity at 332–336 nm (peak of native protein) to that at 320–400 nm. (E) HeLa cells were mock-transfected or transfected with myc-tagged wild-type PDILT or the PDILT cysteine mutants as indicated. Lysates of these transfectants were analyzed by nonreducing (lanes 1–5) and reducing (lanes 6–10) SDS-PAGE. Monomeric PDILT (PDILT-SH) and PDILT in disulfide-linked complexes (PDILT-SS-*) are indicated. Molecular weights in kDa are indicated on the right-hand side. (F) Human insulin, 0.17 mM, was incubated with DTT only (insulin) or DTT with 1 μM enzyme (PDI or PDILT) in PBS. Insulin without DTT (insulin-DTT) was indistinguishable from PBS. The OD600 was monitored over 30 min to follow precipitation of the insulin B chain. (G) Extracts of E. coli expressing ERp27 were incubated with 125I-labeled Δ-somatostatin with (lane 1) or without (lane 2) 2 μg recombinant PDILT. After cross-linking with disuccinimidyl glutarate, the samples were analyzed by SDS-PAGE.