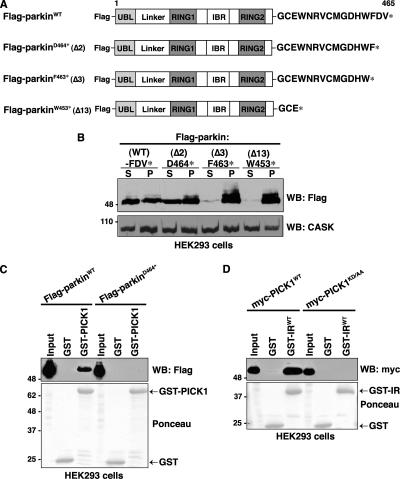
Figure 2.
Identification of critical residues involved in the parkin-PICK1 PDZ-mediated interaction. (A) Schematic representation of Flag-parkin constructs. (B) C-terminal truncations exceeding two amino acids render parkin insoluble. HEK293 cells were transiently transfected with the indicated wild-type and C-terminal deletion constructs of parkin. Cells were harvested, lysed in detergent buffer (0.5% Triton X-100 in TBS), and fractionated by centrifugation, and parkin present in the detergent-soluble (S) and -insoluble (P) fractions was analyzed by Western blotting with anti-Flag antibodies. Deletion of the last two residues of parkin (D464*) does not affect parkin solubility compared with the wild-type protein. However, deletion of the final 3 (F463*) or 13 (W453*) amino acids of parkin renders the protein insoluble to mild detergent. Endogenous CASK levels are shown in the bottom panel as a loading control. (C) Deleting the final two amino acids of the PDZ-binding motif (Flag-parkinD464*) of parkin eliminates binding with PICK1 in vitro. Extracts from HEK293 cells transiently transfected with wild-type or PDZ mutant Flag-parkin were incubated with either GST-PICK1 or GST alone. Binding was assayed by SDS-PAGE and Western blotting with the indicated antibodies. (D) Mutation of the PDZ domain of PICK1 disrupts the interaction with parkin. Extracts from HEK293 cells transiently transfected with wild-type or PDZ mutant myc-PICK1 (myc-PICK1KD/AA) were incubated with the indicated GST fusion proteins. Binding was assayed by SDS-PAGE and Western blotting with the indicated antibodies.