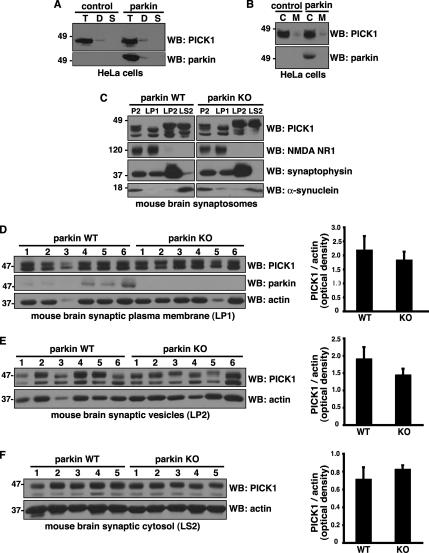
Figure 6.
Parkin does not affect PICK1 subcellular localization. (A and B) Subcellular distribution of endogenous PICK1 in HeLa cells stably expressing Flag-parkin or empty vector control. (A) Cells were sequentially extracted with 0.5% Triton X-100 (T), 1% deoxycholic acid (D), and 2% SDS (S). Equal volumes were loaded and immunoblotted with the indicated antibodies. Both PICK1 and parkin were predominantly found in the Triton X-100–soluble fraction with a small amount in the Triton X-100–resistant, deoxycholic acid–soluble fraction. (B) Cells were lysed mechanically without detergent, and the cytosolic (C) and membrane fractions (M) were separated by centrifugation. Similar amounts of protein (volume ratio of 4:1 cytosol:membrane fraction) were loaded and immunoblotted with the indicated antibodies. Both PICK1 and parkin were predominantly found in the cytosolic fraction. Importantly, parkin expression did not change endogenous PICK1 distribution using either the detergent (A) or mechanical (B) fractionation procedure. (C) Distribution of PICK1 in subsynaptic fractions prepared from parkin wild-type and knockout mouse brain synaptosomes (P2). Immunoblotting showed a similar distribution pattern of PICK1 in parkin wild-type and knockout fractions. Antibodies against NMDA NR1, synaptophysin, and α-synuclein were used as markers of synaptic plasma membrane (LP1), synaptic vesicle (LP2), and synaptic cytosolic (LS2) fractions, respectively. (D–F) Quantification of PICK1 levels in synaptic plasma membrane (D), synaptic vesicle (E), and synaptic cytosol (F) from parkin wild-type and knockout mice. Equal amounts of protein were loaded from the indicated number of mice and immunoblotted with the antibodies shown. Densitometric analyses of endogenous PICK1 band intensities, normalized to actin, used as a loading control, are presented (right) as mean ± SEM. Student's t test reveals no significant differences between parkin wild-type and knockout samples.