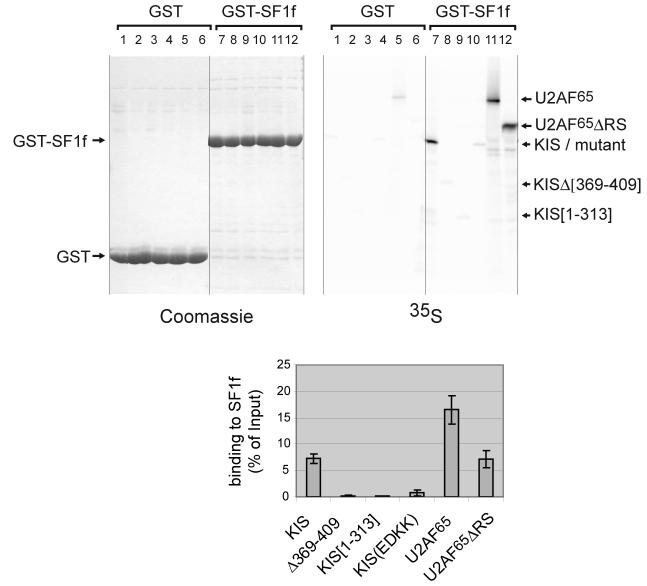
Figure 1. KIS interaction with SF1 in vitro.
Various methionine labelled forms of KIS and U2AF65 were tested for their binding to GST-SF1[1-255] (GST-SF1f) in a pull-down assay (top right, lanes 7-12). Lane 7: wildtype KIS; lane 8: KIS with a deletion within its UHM domain, KIS[Δ369-409]; lane 9: KIS lacking its UHM domain, KIS[1-313]; lane 10: KIS with mutations of E341 and D342 to lysine, KIS(EDKK), lane 11: full-length U2AF65 and lane 12: U2AF65 lacking its RS domain, U2AF65ΔRS. Background binding on GST beads was analysed (lanes 1-6) and 0.5% of starting material was run in parallel to allow quantification (not shown). Mean values of three experiments are presented with standard deviations for binding to SF1f (bottom). The equivalent loading of the beads with GST and GST-SF1f was checked by Coomassie staining of the gel (top left).