FIGURE 2. Sequence of β protein summarizing the limited proteolysis and biotin modification data.
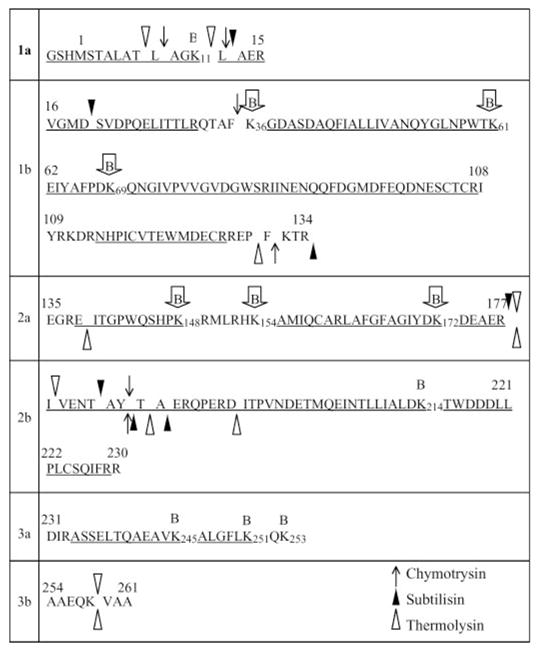
The sequence is divided into segments based on limited trypsin digestion. Region 1 is a stable N-terminal core domain that is resistant to trypsin in the presence and absence of DNA and is divided into 1a and 1b based on cleavage at Lys-15 to generate band D in Fig. 1A. Domain 2 becomes resistant to trypsin in the DNA complex and is divided into 2a and 2b based on cleavage at Arg-177 to generate band C. Domain 3 is the C-terminal “tail,” which is highly susceptible to trypsin both in the free protein and in the complex with DNA, and is further divided into 3a and 3b based on cleavage at Lys-253 to generate band A. For the other three proteases, the symbols above and below the sequence indicate the observed proteolytic cleavage sites in the presence and absence of DNA, respectively. Lysine residues that are observed to be biotinylated are indicated above the sequence with the B symbols, where those enclosed in arrows are protected from biotinylation in the DNA complex. Underlined portions of the sequence indicate the regions covered by the MS/MS analysis. The MS analysis of biotinylated protein did not provide information for Lys-111, Lys-132, Lys-253, and Lys-258.
