FIGURE 5. DNA binding of N-terminal fragments of β protein containing lysine to alanine mutations.
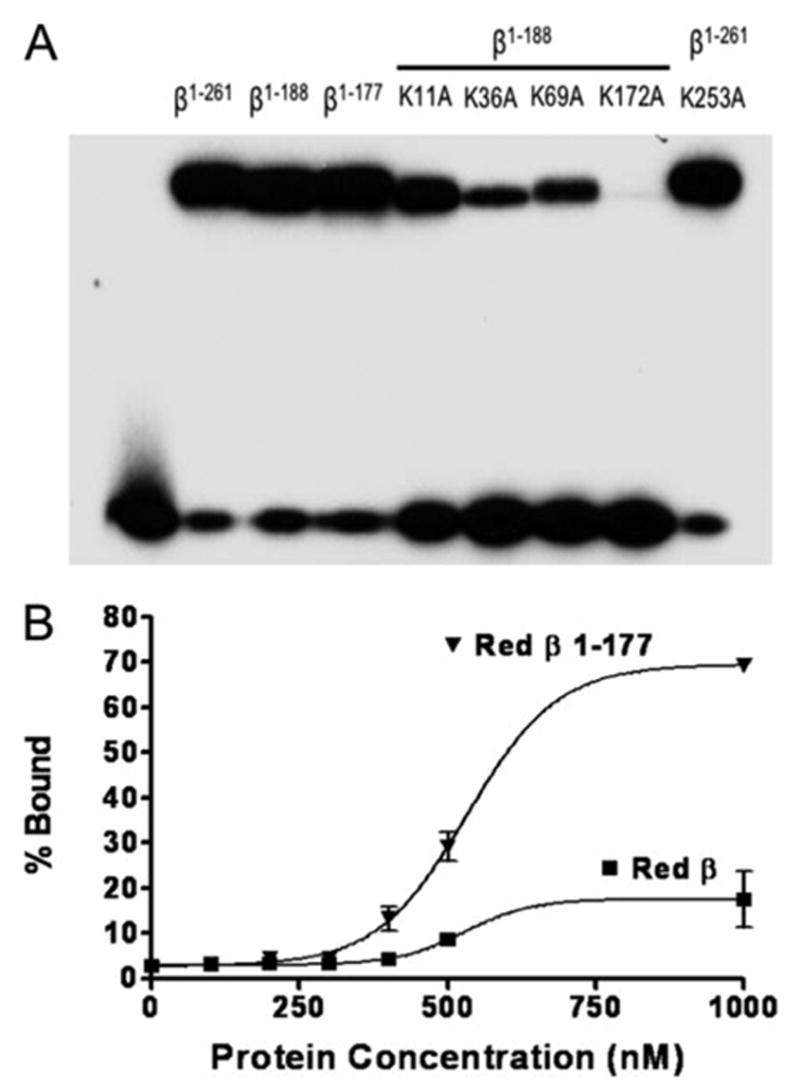
A, the binding of full-length or N-terminal fragments of β protein to sequentially added complementary 33-mer oligonucleotides was carried out as described in “Experimental Procedures.” The band at the top of the gel shows each protein in complex with the 33-mer duplex product of annealing, whereas the band at the bottom corresponds to unbound 33-mer duplex. Notice that the two fragments of β protein (β1–188 and β1–177) bind to DNA about as well as full-length β protein (residues 1–261) and that the lysine to alanine mutants of β1–188 bind less well, with the degree of impairment following the trend K172A > K36A > K69A > K11A. The K253A mutation within full-length β protein (at the far right) does not significantly impair DNA binding. B, binding of full-length β protein and β1–177 fragment to a single 33-mer ssDNA oligonucleotide (33+) was measured using a nitrocellulose filter binding assay (22), as described under “Experimental Procedures.” Notice that the β1–177 fragment binds to the 33+ oligonucleotide with considerably higher stability than full-length β protein.
