Figure 1.
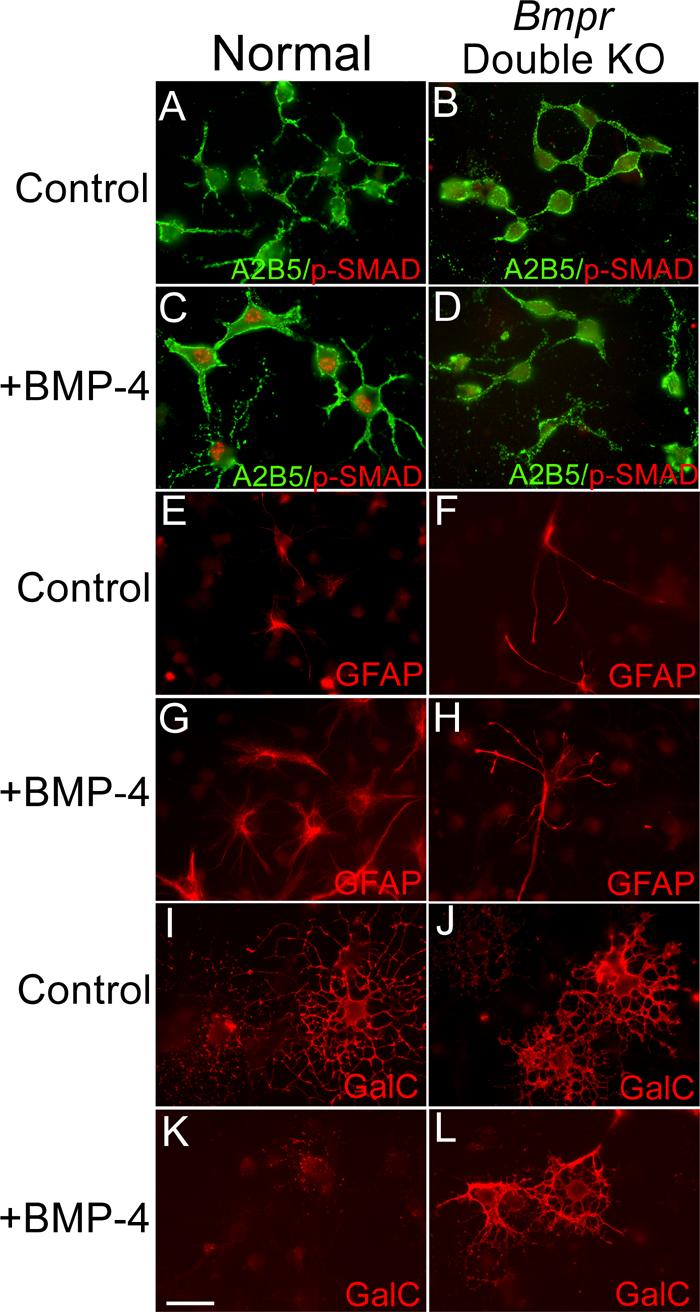
BMP signaling in the oligodendrocyte lineage is disrupted in conditional double knockout Bmpr mice.
Cultures of OPCs were established from brains of P0 normal and double knockout mice. The cultures were placed in differentiation medium, treated with 50ng/ml BMP for 24 hours and then labeled with the A2B5 antibody and antibody to phosphorylated Smad1. Two coverslips were examined from each of three separate preparations. Normal OPCs have only a low background level of phosphoSmad labeling (panel A). Upon BMP treatment, OPCs demonstrate nuclear labeling of phospho-Smad (Panel C). Cultures of OPCs from double mutant animals do not show phospho-Smad labeling with or without BMP treatment (Panels B and D). In addition, some cultures were treated with 50ng/ml BMP for 2 days and then processed for immunofluorescence using an antibody to detect GFAP and the 01 antibody to detect GalC. Two coverslips were examined from each of three separate preparations. In the cultures from normal animals without BMP, only a few contaminating cells are GFAP+ (panel E) whereas many cells express GalC and demonstrate the morphology of mature oligodendrocytes (panel I). When cells from the normal cultures are treated with BMP, many cells express GFAP (panel G) and GalC expression is inhibited (panel K). In the cultures from the Bmpr double knockout animals, BMP treatment has no effect; the cultures contain few GFAP+ cells in general (panel F) and BMP treatment does not increase this number (panel H). GalC+ process-bearing oligodendrocytes are present in control as well as BMP treated cultures (panels J and L). The scale bar in K equals 30 μm.
