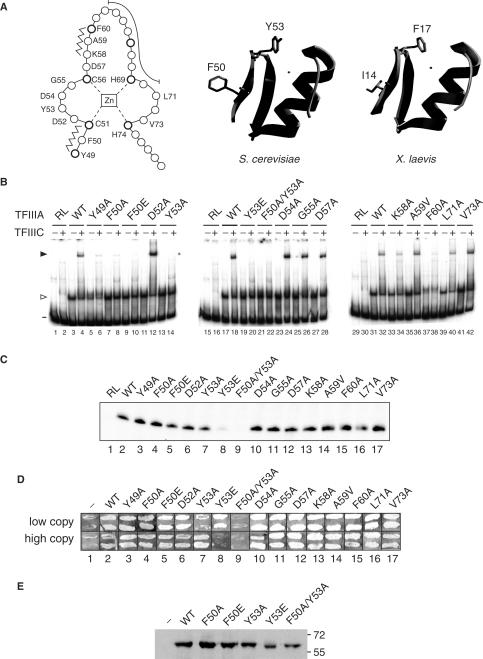
Figure 3.
TF activity of alanine-scanning mutants of the first zinc finger of TFIIIA. (A) A linear representation of the first zinc finger of TFIIIA from S. cerevisiae is on the left. Amino acids are represented by circles, with the position of conserved residues of the zinc finger motif shown in bold. The zinc ion, which is tetrahedrally coordinated by two cysteine and two histidine residues, is boxed in the center of the diagram. Residues that form the short anti-parallel β-sheet of the zinc finger domain are indicated by the zigzag lines, while residues that make up the DNA-binding α-helix are shown by the long curved line. Zinc-coordinating residues and residues that were mutated are identified by their single-letter codes. A ribbon representation of a 3D homology model of the first zinc finger of yeast TFIIIA (residues Y49 to A79) is in the middle. This modeled structure was obtained with the use of the SWISS-MODEL server (63,64) and with the coordinates of the first zinc finger of TFIIIA from X. laevis as the template (17; ExPDB entry 1tf3A; shown on the right). The side chains of F50 and Y53 of the yeast protein and the side chains of the corresponding residues, I14 and F17, of the amphibian protein are shown. The zinc ion, shown by the small circle, is positioned in the yeast structure according to its coordinates in the amphibian protein. (B) Abilities of versions of TFIIIA with alanine-scanning mutations to bind to the 5 S RNA gene and to recruit TFIIIC to the TFIIIA–DNA complex as assessed by EMSA. See Figure 2E for details. The version of TFIIIA used in each reaction is indicated below the gels; RL, reticulocyte transcription–translation reactions not programmed to synthesize TFIIIA; WT, wild-type TFIIIA. The presence (+) or absence (–) of TFIIIC is indicated above the gel. The positions of free DNA (minus sign), TFIIIA–DNA complexes (arrowhead) and TFIIIC–TFIIIA–DNA complexes (solid arrowhead) are shown on the left. (C) Abilities of mutant versions of TFIIIA to support in vitro transcription of the 5 S RNA gene. See Figure 1D for details. (D) Abilities of mutant versions of TFIIIA to support cell viability. See Figure 1G for details. (E) Assessment by western blot analysis of in vivo expression of selected mutant versions of TFIIIA from the ΔpG3 series of plasmids. See Figure 1G for details.