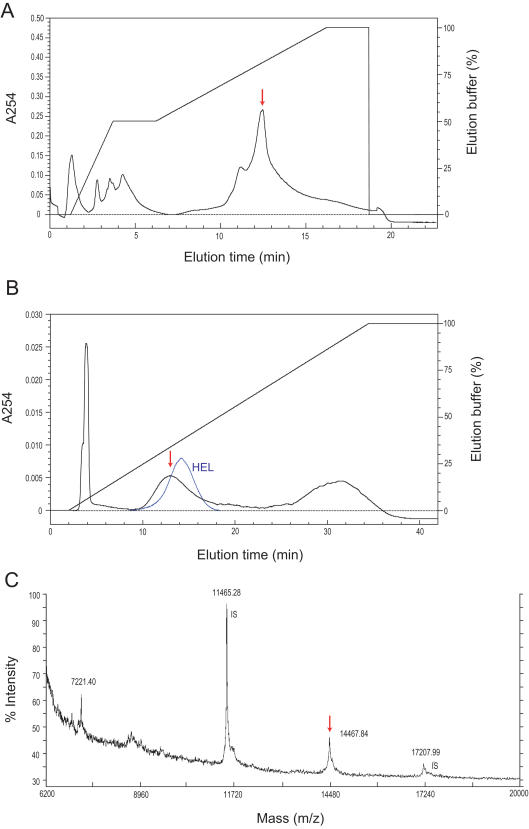
Figure 3. Chromatography and MALDI-TOF MS analyses.
A, UNO Q-1 anion-exchange chromatography elution patterns of the TERP. The active peak is indicated by an arrow. Lysozymes bind with coexisting anionic proteins in the Tris-HCl buffer (pH 8.2) and thus are eluted as the absorbed fraction, although lysozyme itself is a cationic protein. B, Methyl HIC column chromatography. The active peak is indicated by a red arrow. The elution pattern of hen egg lysozyme (HEL) is indicated by the blue line. C, MALDI-TOF MS spectrum of the purified TERP. Ions at m/z 14467 and 7221 (divalent ion) were identified as the target protein. Ions at m/z 11465 and 17207 were the dimer and trimer of insulin used as the internal standards (IS), respectively.