Scheme 1.
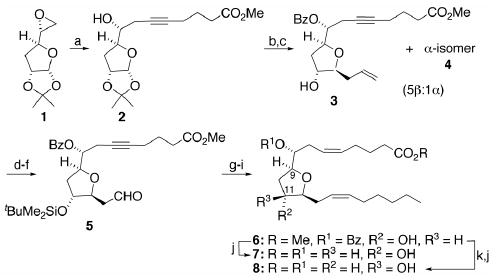
Reagents and conditions: (a) 5-hexynoic acid, n-BuLi (2 equiv), HMPA, 5°C for 1 h, then 23°C for 12 h; CH2N2, 5% MeOH/Et2O, 23°C, 1 h, 60–65%; (b) PhC(O)Cl, DMAP, Et3N, CH2Cl2, 23°C, 12 h, 97%; (c) Me3SiCH2CH=CH2/BF3·Et2O, CH2Cl2, 23°C, 18 h, 82% (α-/β-isomers combined); (d) t-BuMe2SiCl, ImH, DMF, 50°C, 12 h, 95%; (e) OsO4 (2 mol %), NMO, t-BuOH, 23°C, 12 h; (f) NaIO4/SiO2, CH2Cl2, 23°C, 1.5 h; (g) H3C(CH2)5PPh3Br, NaN(SiMe3)2, PhCH3/THF (1:1), −90°C for 0.5 h, then warm to 23°C overnight, 70% over three steps; (h) Ni(OAc)2, NaBH4, (H2NCH2)2, H2 (1 atm), EtOH, 23°C, 5 h, 85%; (i) n-Bu4NF, THF, 23°C, 12 h, 70%; (j) LiOH, THF/H2O (3:1), 92%; (k) DEAD/PPh3/PhCO2H, THF, 1 h, 0°C, 93%.
