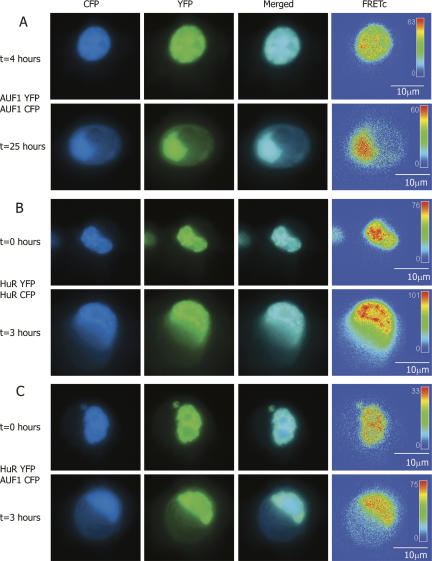
FIGURE 2.
FRET-based detection of p37AUF1/p37AUF1, HuR/HuR, and p37AUF1/HuR protein pair interactions in live cells. DDT1-MF2 cells were transiently transfected with pairs of complimentary, fluorescently labeled constructs of HuR and/or p37AUF1. Transfected cells were treated with anisomycin to stimulate nuclear/cytoplasmic shuttling of mRNA binding proteins. (A) p37AUF1-eYFP and p37AUF1-eCFP interaction. After 4 h of treatment with anisomycin, p37AUF1 remains localized to the nucleus (top row) and exhibits colocalization (merged channel column) detectable by FRETc, which is indicative of a stable homodimeric interaction (top row, FRETc column). After 25 h of anisomycin treatment, cytoplasmic shuttling of p37AUF is readily apparent (bottom row). The p37AUF1 present in the cytoplasm colocalizes, which is detectable by the merged channel view and the FRETc signal present in both the nucleus and cytoplasm. (B) HuR-eYFP and HuR-eCFP interaction. At time zero, HuR is present in the nucleus, and exhibits colocalization and a robust FRETc signal (top row). Anisomycin causes rapid shuttling of HuR to the cytoplasm, and a FRET-detectable, stable interaction between HuR is present in both the nucleus and cytoplasm (bottom row). (C) HuR-eYFP and p37AUF1-eCFP interaction. At time zero, p37AUF1 and HuR are present in the nucleus, colocalize, and display a FRETc signal indicative of a stable heteromeric interaction (top row). Treatment with anisomycin causes shuttling of both HuR and p37AUF1 into the cytoplasm. HuR and p37AUF1 colocalize in the cytoplasm and exhibit a FRET-detectable interaction in both the nuclear and cytoplasmic compartments. FRETc images are shown in thermal gradient pseudocolor. The scale bars in the FRETc image applies to all images in the same row.