Figure 3.
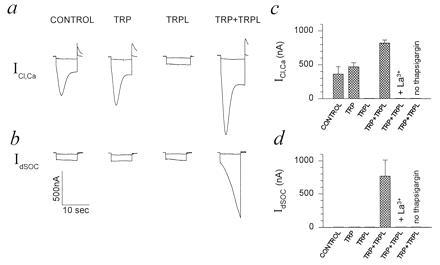
Functional coexpression of TRP+TRPL in Xenopus oocytes produced a capacitative Ca2+ entry system revealed by ICl,Ca and IdSOC. Shown is a single experiment, employing several oocytes from a single frog that were maintained and treated together. (a) Measurements of currents (ICl,Ca) in thapsigargin-treated oocytes (1 μM in Ca2+-free solution for 1.5–2 hr). ICl,Ca was activated by stepping the holding voltage from −10 mV to −30 mV (upper traces) and to −120 mV (bottom traces) to show the relatively small instantaneous leak current in solution containing 1 mM Ca2+ (see Fig. 2). Oocytes were injected with cRNA 5 days before the measurements. (b) Measurements of IdSOC were carried out as described in a. The same oocytes were perfused with Ca2+-free ND96 solution (10 mM Mg2+). In some of the measurements, 2 mM EGTA was injected into the oocytes 1–2 hr before the recordings, but no significant effect on IdSOC was found. IdSOC was totally and reversibly blocked by addition of 1 mM La3+ to the perfusate (c and d). IdSOC was also blocked reversibly by 500 μM, but not by 50 μM, La3+ (n = 12). (c and d) Histograms summarizing the results from all the oocytes of the same experimental run of a and b. Five to 10 oocytes were used for each of the experimental groups of a and b. The histograms present the mean and SEM of the peak ICl,Ca (c) and maximal IdSOC (d) measured at −120-mV holding potentials after the instantaneous leak currents were subtracted from all current traces. The TRP+TRPL group was significantly different from the other oocyte groups of c (P < 0.01). The control and TRP groups were not significantly different (P > 0.05).
