Figure 3.
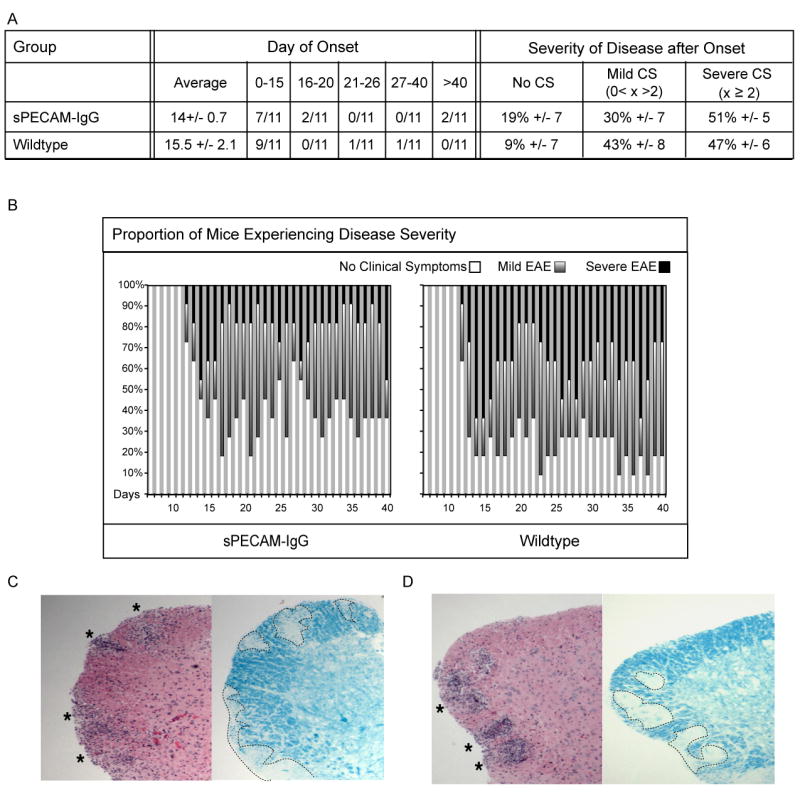
sPECAM-Fc mice with 2-9 μg/ml in serum do not have different onset or severity of EAE symptoms than wildtype mice. A. Shown is average day onset, plus or minus standard error of the mean, and the number of mice per group to experience onset within the given time frames. The average percentage of time during the fifteen days after onset that mice in each group experienced no clinical symptoms, mild EAE with clinical symptoms greater than zero and less than 2, and severe EAE with clinical symptoms of 2 and above is shown. B. Graphical representation shows the proportion of mice in the group on each day experiencing no onset or no clinical symptoms (white portion of bar), mild EAE (gray portion of bar), or severe EAE (black portion of bar). The protection afforded by the low levels of sPECAM-Fc did not reach statistical significance. (C) Wildtype and (D) sPECAM-Fc mice. Spinal cords were removed 40 days post EAE induction. Paraffin-embedded sections were stained with H&E or LFB to detect infiltrating mononuclear cells and demyelinated areas, respectively. Both wildtype and sPECAM-Fc transgenic mice showed visible signs of infiltration and demyelination in the spinal cord.
