Abstract
The importance of sulfate respiration in the microbial mat found in the low-sulfate thermal outflow of Mushroom Spring in Yellowstone National Park was evaluated using a combination of molecular, microelectrode, and radiotracer studies. Despite very low sulfate concentrations, this mat community was shown to sustain a highly active sulfur cycle. The highest rates of sulfate respiration were measured close to the surface of the mat late in the day when photosynthetic oxygen production ceased and were associated with a Thermodesulfovibrio-like population. Reduced activity at greater depths was correlated with novel populations of sulfate-reducing microorganisms, unrelated to characterized species, and most likely due to both sulfate and carbon limitation.
Cyanobacterial microbial mats are layered communities in which the combination of microbial activity and environmental gradients contributes to a fine vertical stratification of microbial populations and activities. They are self-sustaining communities that support all major biogeochemical cycles and are thought to be analogous to some of the earliest communities on Earth (9, 11). These attributes have made microbial mats attractive models for better understanding both the evolution and the ecology of microbial systems supporting the processes that now sustain our biosphere.
Modern mat communities occur primarily in environments in which temperature or salinity suppresses grazing by higher eukaryotes (22, 38, 45). The mats that develop in low-sulfate, moderately thermophilic (50 to 72°C), moderately alkaline (pH ∼8), siliceous hot spring effluents in Yellowstone National Park (YNP) are among the best characterized, and analyses of these systems have provided an increasingly complete view of their diversity and ecology (41-43). Most past studies of these mats have focused on the contribution of cyanobacteria (42), key biogeochemical players through both primary production and the generation of oxygen used by highly active populations of aerobes near the surface.
Although all major anaerobic processes are also recognized to be present in the deeper, anoxic regions of these mats, much less attention has been directed toward resolving their contribution to mat biogeochemistry. In particular, since these mats develop in low-sulfate springs, the role of sulfate-respiring prokaryotes (SRP) has received relatively little attention (13, 33, 48). However, a low concentration of sulfate can support a highly active population of SRP in communities that sustain populations mediating both the oxidizing and the reducing pathways of the sulfur cycle. This has been observed in certain freshwater lakes (21) and in hot springs supporting the growth of photosynthetic biofilms (15, 16). The importance of sulfate respiration in low-sulfate environments is also of considerable significance for understanding the biogeochemistry of early Earth, in which oxidized sulfur species were much less abundant than today (6, 20). The present study combined radiometric rate measurements, chemical profiling, and molecular phylogenetic analyses to investigate the role of sulfate respiration in the microbial mat found in Mushroom Spring, one of these low-sulfate thermal spring effluent environments. This study represents the first comprehensive investigation into the changing daily role of sulfate respiration and is the first to link activity measurements to phylogenetic identity in these low-sulfate microbial mats. It revealed a highly active sulfate-reducing community composed of only a few dsrAB genotypes, especially active near the surface during the switch from net photosynthetic to net respiratory conditions at the end of the day.
MATERIALS AND METHODS
Study site and sample collection.
During the summers of 2000, 2001, and 2003, in situ spring water pH and temperature in the effluent channel of Mushroom Spring, a thermal spring located in the lower geyser basin of YNP, WY, were measured using a pH 330 combined pH and temperature probe (WTW, Ft. Myers, FL). Samples between 56 and 60°C, a temperature range over which a 2- to 4-cm-thick microbial mat was growing on top of the siliceous substrate, were collected. Replicate 1-cm-diameter glass cores were used to sample the mat for nucleic acid extraction, sulfate determinations, and sulfate reduction rate (SRR) measurements. Cores for nucleic acid extraction were immediately frozen on dry ice and returned to the laboratory, where they were stored at −80°C until extraction.
Sulfate measurements.
In 2000, bulk porewater sulfate concentrations ([SO4] values) were determined for 1-cm cores by capillary electrophoresis using a Quanta 2000 capillary ion analyzer (Waters Corp., Milford, MA) equipped with a negative power supply. In 2001 and 2003, the sulfate was analyzed by ion chromatography. In June 2001, a single set of core samples (n = 4) was collected at 09:00 with a 30-ml syringe with the tip removed. Cores were sliced with a sterile razor blade in 2-mm slices for the first 4 mm and into 4-mm slices below that until fully sampled (typically 2 to 3 cm). On 6 and 7 September 2003, diel samples were taken at 20:00, 23:00, 08:35, and 11:41, in parallel with the first four SRR sampling points (see below). Replicate cores (n = 3 to 5) were sampled with a 20-ml syringe with the tip removed and sliced using very fine monofilament fishing line (4 lb) at the following intervals from the surface of the mat: 0 to 2 mm, 2 to 4 mm, 4 to 6 mm, 6 to 8 mm, 8 to 10 mm, and 10 to 15 mm. In both 2001 and 2003, core slices were placed into a preweighed 15-ml centrifuge tube preloaded with 1 ml of 0.1% ZnCl2 and frozen on dry ice. The samples were returned to the University of Southern Denmark, where the sulfate was analyzed using an ICS-2000 anion exchange chromatograph (Dionex). The eluents were 3.5 mM Na2CO3 and 1 mM NaHCO3, and the flow rate was 2 ml min−1. The detection limit was 1 μM sulfate.
Diel SRR assays.
During July 2000, two sets of triplicate core samples were taken during daytime and incubated in the hot spring effluent following vertical injection of 10 μl (0.37 MBq, 10 μCi) of Na235SO4 (specific activities, 38.8 to 59.2 TBq mmol−1) through the center of the core with a Hamilton syringe. One set was left under ambient-light conditions, while another set was incubated in the dark to control for light effects. In July 2001 and September 2003, triplicate core samples were taken at multiple time points over the diel period. To minimize the effect of reoxidation of sulfide generated (19), 5-min incubation times were used in all experiments. Following incubation, the cores were immediately removed from the spring and sliced, and further sulfate reduction was stopped and the sulfide bound by placing each slice into a 2-ml screw cap tube containing 0.5 ml of 20% zinc acetate solution and freezing it on dry ice. Slicing was performed at an ambient-air temperature by using the monofilament line over the following intervals: 1 mm for the first 4 mm, 2 mm for the next 6 mm, 0.5 cm for the next cm, and 1 cm below that until the core was fully sampled (usually 2 to 3 cm). The entire slicing/killing procedure typically took approximately 5 min; however, the surface slices were taken first and reactions were stopped immediately. SRRs were calculated according to the equations of Fossing and Jørgensen (17), using an incubation time of 5 min. This may result in our calculated SRRs being slight overestimates of specific rates for some of the deeper-sliced samples; however, consistent sampling practices across replicate diel sampling time points and across years allowed us to assess relative diel changes in rates with confidence. Sulfate reduction was confirmed to be due to bacterial activity by using triplicate cores that were incubated with 10 μl of 66 mM sodium molybdate injected immediately after the radiolabeled substrate was added. These rates were never higher than 6% of the midday rates for each layer. Additionally, a nonincubation control core was stopped by addition of 0.5 ml of 20% zinc acetate and freezing on dry ice immediately after injection with Na235SO4. These nonincubation control values were subtracted from rates for each layer and were never greater than 10% of the lowest rates measured. In 2000, samples were analyzed for sulfate reduction by using the single-step chromium reduction method (17). In the calculation of SRRs for all three years, [SO4] values from 2003 were used to better reflect the decrease in sulfate with depth and the diel change in [SO4]. The daytime [SO4] values measured in 2000 and 2001 were highly consistent. Because of the large number of samples collected in 2001 and 2003, the modified shaking protocol of Ulrich et al. (37) was used for analyzing the SRRs of these samples. Similar rates in replicate cores processed using both methods were found (unpublished observation).
Microelectrode profile measurements.
Oxygen microelectrode profile measurements were made in parallel with SRR incubations in 2000 and 2001, using a Clark-style oxygen microsensor with a 10-μm tip manufactured by Diamond General (Ann Arbor, MI) connected to a Keithley 485 picoammeter. Vertical profiles were made by sequentially moving the electrode, using a micromanipulator, down into the mat and recording the electrical current. In 2003, both oxygen and sulfide profiles were made using Clark-style microsensors (Unisense, Aarhus, Denmark).
Hydrogen sulfide profile data from the late evening, after oxygen concentrations in the mat had dropped and sulfide was detectable in the mat, were used to calculate specific SRRs by using diffusion coefficients and parabolic function fitting as described in Kühl and Jørgensen for biofilms (26). Rates were calculated as the product of the diffusion coefficient and the coefficient of X2 for the parabolic function calculated from the H2S profile. As reported in the Kühl and Jørgensen study, we multiplied the diffusion coefficient by 80% to account for the reduced diffusion within the mat compared to that in the water environment in which the coefficient was originally measured (26). However, we used a higher sulfide diffusion coefficient of 3.87 × 10−5 (35) to account for the higher temperature in the hot spring mat environment.
Light measurements.
Irradiance was measured in 2003 using an LI-1000 data logger with an LI-190 quantum sensor (Li-Cor, Lincoln, NE).
Nucleic acid isolation.
Total microbial mat DNA was extracted in both 2000 and 2001 from the 0- to 1-mm layer, dominated by cyanobacterial pigments, and from the 1- to 2-mm layer, rich in carotenoid pigments of single-core samples. In 2001, an additional slice below the oxycline between 15 and 18 mm was sampled from the same core. All layers were excised with a sterile razor blade, and extractions were performed using an UltraClean Soil DNA kit (MoBio, Solana Beach, CA), following the manufacturer's instructions.
PCR amplification, cloning, and sequencing.
PCR amplifications were performed using modified versions (Table 1) of the DSR1f and DSR4r primers, targeting a large fragment of the dissimilatory sulfite reductase gene (dsrAB) (39). PCR mixtures contained 2 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate, 2 μl of 10× PCR buffer, 1.5 U Platinum Taq DNA polymerase (Gibco/Life technologies, Rockville, MD), a DNA template at 0.04 to 0.4 ng/ml, and 10 pmol each of both primers in a total volume of 20 μl. Because the optimal annealing temperatures of the degenerate dsrAB primers can vary for different templates, amplifications were carried out with a gradient thermocycler (ThermoHybaid, Franklin, MA) programmed as follows: an initial denaturation for 5 min at 95°C, followed by 30 cycles at 95°C for 30 s, exposure to a gradient of annealing temperatures from 50°C to 65°C for 30 s, and elongation at 72°C for 1 min. The reaction was completed with a final elongation step at 72°C for 10 min. The best amplified dsrAB gene products were ligated into the pCR4-TOPO cloning vector and transformed into ONE SHOT competent Escherichia coli cells, following the manufacturer's directions (TOPO TA cloning kit for sequencing; Invitrogen/Life Technologies, Carlsbad, CA). Cloned inserts were first screened for proper size (ca. 1,800 bp) by amplification using M13 vector primers and then sequenced with M13F/R vector primers. After initial screening of clones for sequence similarity, full-length sequences from select clones from each major clade were obtained using sequence-specific internal primers designed for this study (Table 1). Sequencing reactions were performed using a DYEnamic ET dye terminator cycle sequencing kit (Amersham Biosciences, Piscataway, NJ) and run on a Megabace 1000 system (Amersham Biosciences) at the University of Washington Center for Marine Biotechnology.
TABLE 1.
Sequences for primers targeting dsrAB genes used in this study
| Primer | Sequence (5′-3′) | Source or reference |
|---|---|---|
| DSR1f | ACSCACTGGAARCACG | 39a |
| DSR4r | GTGTAKCAGTTDCCRCA | 39a |
| DSRINTMSF1 | CAYTGTATGCATTGTATT | This study |
| DSRINTMSR1 | TTAGCTATATGAACCATT | This study |
| DSRINTMSF2 | TACAACGAGGACTGCACC | This study |
| DSRINTMSR2 | CTCTCGGATACGTGGACC | This study |
| DSRINTMSF3 | AAGATAAATGACGGTGAT | This study |
| DSRINTMSR3 | GATACTTCCATTTTCCAT | This study |
| DSRINTMSF4 | GACAATAGCAGCTGCCGT | This study |
| DSRINTMSR4 | CCACTTTCGGCAGTGTGA | This study |
Modified from the original published primer.
Phylogenetic and statistical analyses.
Phylogenetic analyses were performed on alignments of the inferred amino acid sequences of amplified dsrAB DNA sequences. Alignments of dsrAB were performed using the alignment tool of the ARB software package (http://www.arb-home.de) (28) and comparing the sequences to those in our own dsrAB database. Alignments were then refined manually by visual inspection. Regions of uncertain alignment or containing missing data were excluded from analysis. Phylogenetic trees were constructed using neighbor-joining and parsimony methods implemented in PAUP* v. 4.0b10 software (D. L. Swofford, Sinauer Associates, Sunderland, MA). Bootstrap resampling of the neighbor-joining and parsimony trees was performed using 1,000 replicates for each.
Regression analyses and one- and two-way analyses of variance (ANOVA) were performed using StatView v. 5.0 (SAS Institute, Cary, NC). Planned pairwise comparisons were performed using Fisher's protected-least-significant-difference (PLSD) method.
Nucleotide sequence accession numbers.
Sequences were submitted to GenBank and assigned the accession numbers EF429274 through EF429285.
RESULTS
Hot spring conditions.
Physical and chemical conditions in Mushroom Spring and associated effluent channels were consistent among the 2000, 2001, and 2003 sampling dates and consistent with reports dating back to at least 1988 (2). The pH levels in the outflow channels were 8.1 to 8.3, and thick (2- to 4-cm) mats were consistently found between 56 and 60°C. Sulfate levels in the Mushroom Spring microbial mat were very low. During the 2000 sampling period, the bulk porewater [SO4] in the mat was 193 μM. In 2001, the value was 184 μM in the overlying water, but the mean porewater concentration within specific mat slices was 102 μM in the upper 1 mm and decreased rapidly to ∼10 to 15 μM within 1 cm (Fig. 1). In 2003, repeated sampling in parallel with SRR experiments (see below) was performed to determine whether depth profiles for porewater [SO4] varied over the diel period. The [SO4] was 202 μM in the spring water, but two-way ANOVA showed that [SO4] decreased significantly with depth (P < 0.0001) in the Mushroom Spring microbial mat, from a mean of 137 μM in the upper 2 mm down to 22 μM SO42− between 10 and 15 mm (Fig. 2). Pairwise comparisons among depth intervals confirmed that mean porewater [SO4] values were significantly higher in the upper 0- to 2-mm and 2- to 4-mm layers than for any lower-depth interval (Fisher's PLSD; P < 0.0001 and P < 0.005, respectively). Mean sulfate values changed significantly over the diel cycle, with the highest levels at midday and the lowest levels at nighttime (P = 0.0104) (Fig. 2). A significant depth-by-time interaction term (P = 0.0038) reflects a less steep decrease in sulfate between the 0- to 2- and 2- to 4-mm intervals during the midday sampling time than at other sampling times. These diel changes may reflect the changes in sulfate consumption due to sulfate respiration (see below).
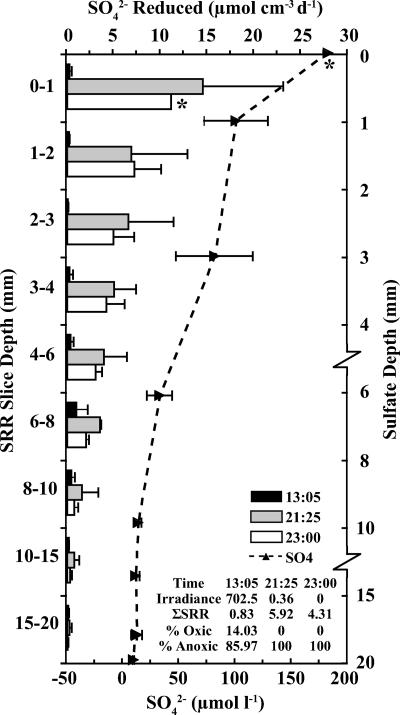
FIG. 1.
Vertical profiles of SRRs at 13:05 (black bars), 21:25 (gray bars), and 23:00 (white bars) on 10 July 2001 and sulfate concentrations (dashed lines) from 28 June 2001. Note breaks and changes in scale on the sulfate depth axis. An error bar denotes 1 standard deviation (n = 3 or 4) or a range (n = 2). *, n = 1. Integrated SRRs (μmol cm−2 day−1 [d−1]), overall depths, and proportions of total SRRs above (% oxic) and below (% anoxic) the oxycline calculated for each sampling time are in the inset.
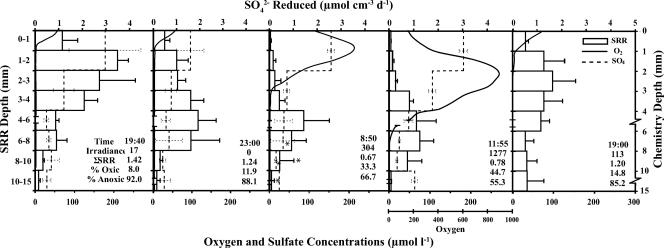
FIG. 2.
Vertical profiles of SRRs (hollow bars; an error bar denotes 1 standard deviation [n = 3] or a range [*; n = 2]), oxygen concentrations measured using microelectrodes (solid lines), and sulfate concentrations (dashed lines; an error bar denotes 1 standard deviation [n = 3 to 5] or a range [*; n = 2]) for samples collected in the Mushroom Spring microbial mat on 6 and 7 September 2003. Note breaks and changes in scale on the chemistry depth axis. Sampling times, means for irradiance measurements (photosynthetically available radiation, μE m−2 s−1), integrated SRRs (nmol cm−2 day−1), overall depths, and proportions of total SRRs above (% oxic) and below (% anoxic) the oxycline are in the inset. Note the different scale for the oxygen profiles for the 11:55 sampling point. No sulfate determinations were made for the 19:00 sample on day 2.
Diel cycles of sulfate respiration.
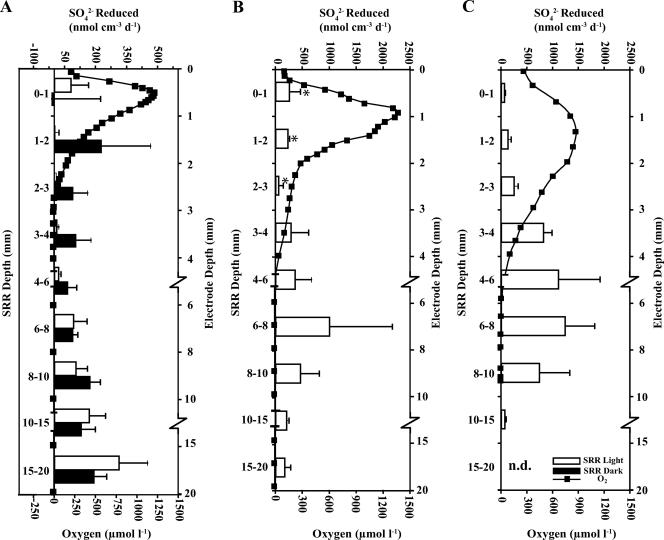
Significant daytime SRRs were measured in the mat during the initial 2000 sampling trip, revealing low but greater-than-nonincubation-control rates near the surface, increasing below the oxycline (ca. 3 mm) to a peak between 15 and 20 mm and declining below that (Fig. 3A). Incubations under dark conditions resulted in a significant increase in mean specific SRR (7.3 versus 143 nmol cm−3 day−1; one-way ANOVA; P = 0.0192) for the upper (0- to 4-mm) zone of the mat, whereas at the permanently anoxic depths below 4 mm, mean SRRs were nearly identical in light and dark (125 versus 128 nmol cm−3 day−1; P = 0.9409). Similar daytime results showing low sulfate respiration activity in the presence of photosynthetic oxygen were detected in subsequent sampling trips in 2001 and 2003, although the depths with peak SRRs in those years were shallower at ca. 6 to 8 mm, with the SRRs decreasing below that depth to near zero (Fig. 3).
FIG. 3.
Vertical profiles of SRRs (open bars) and oxygen concentrations measured using microelectrodes (boxed line) from daytime sampling points in July 2000 (A), July 2001 (B), and September 2003 (C). Black bars in panel A show results from dark control incubations in 2000. An error bar denotes 1 standard deviation (n = 3). Note breaks and changes in scale on the electrode depth axis. n.d., no SRR data.
In both 2001 and 2003, rate measurements at different depths were performed periodically over the diel period, allowing a more comprehensive view of the dynamics of SRR in this system (Fig. 1 and 2, respectively). In both years, highly significant changes were detected with two-way ANOVA of SRR with depth (P < 0.001) and time of day (P < 0.001). Significant depth-by-time interaction terms (P = 0.0068 and P < 0.001, respectively) indicate that the depth profiles changed over the course of the day. In general, SRRs were most variable in the upper 4 to 6 mm of the mat and lowest during daytime hours when photosynthetically generated oxygen was at or above saturation, peaking during early evening hours after oxygen was consumed in most of the mat and remaining high a few hours later in complete darkness (Fig. 1 and 2).
Evening- and nighttime-specific SRR values were as much as 27 to 50 times higher than midday values for a given millimetric interval. In contrast to the midday SRR peak found deep in the mat as described above, rates during the dusk and nighttime points were highest at or just below the surface, decreasing monotonically below that in parallel with declining [SO4] values (Fig. 1 and 2). Evening and nighttime SRRs averaged over the upper 6 mm of the mat were significantly higher than dawn (2003) or midday (both 2001 and 2003) rates (Fisher's PLSD; P < 0.05 for all). In contrast to these near-surface values, mean SRRs for layers below 6 mm did not significantly change over the diel period (P > 0.05 for both years).
A similar daily pattern was found when depth-integrated SRRs (μmol cm−2 day−1) for entire cores were calculated (see inset values in Fig. 1 and 2). In 2001, integrated rates for dusk and nighttime samples were significantly higher than those for the midday samples (Fisher's PLSD; P < 0.01 for both). In 2003, integrated rates for the first evening samples were significantly higher than those for both the dawn and the midday sampling periods (Fisher's PLSD; P < 0.05 for both). The nighttime and the second evening samples had higher average integrated rates, but these differences lacked statistical significance. Despite the significant depression of SRRs above the oxycline (∼6 mm), 14 to 45% of the integrated sulfate respiration activity occurred in this portion of the mat at midday. Therefore, significant sulfate respiration occurred even in the presence of oxygen.
In 2003, the relationship between SRR and [SO4] was assessed. During the evening transition period, in layers without any oxygen (below 1 mm), there was a significant positive correlation between [SO4] and SRR (R2 = 0.479, P = 0.005) (panel 1 in Fig. 2), whereas at nighttime, characterized by diminished levels of sulfate at the surface (panel 2 in Fig. 2), no correlation between SRR and [SO4] was found (R2 = 0.007, P = 0.7124).
Diel changes in microelectrode profiles.
Oxygen levels changed predictably throughout the diel period, increasing rapidly at sunrise, reaching supersaturation during the daytime, when high irradiance drives cyanobacterial photosynthesis, and dropping to zero in all but the upper millimeter by evening, when heterotrophic consumption dominates (Fig. 2). The peak oxygen levels measured in 2000 and 2001 during July were ca. 35 to 55% higher than the September 2003 levels, but the depth of oxygen penetration was somewhat deeper in 2003 (Fig. 3).
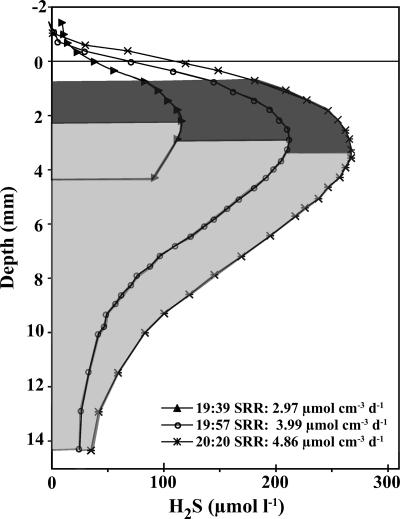
Hydrogen sulfide levels were measured during the transition period during the second evening period in 2003. H2S profiles were very consistent with the corresponding evening patterns of SRR described above; sulfide levels increased to a peak at a few millimeters of depth and then declined below that (Fig. 4). There were >2-fold increases in sulfide concentrations calculated from profiles made between 19:39 and 20:20. Microelectrode depth profiles showed peak H2S concentrations in the mat just below 2 mm of depth at 19:39, closely mirroring the peak SRRs measured during evening sample periods. Peak H2S levels shifted downward in the mat during subsequent measurements until they peaked between 3.25 and 3.5 mm at 20:20. Specific SRRs calculated from the sulfide profiles by using the parabolic fitting approach increased from 2.97 to 4.86 μmol cm−3 day−1 as H2S levels increased during the evening. These values represent total rates calculated for the depths over which sulfide increased in the upper portion of the parabolic curve (see the darker shaded area in Fig. 4). The calculated SRR values were 86 to 140% of the highest radioisotopically determined specific SRRs for evening periods for 2003.
FIG. 4.
Vertical profiles of H2S levels calculated from microelectrode profiles made between 19:39 and 20:20 on 7 July 2003. Dark gray areas under the curve represent the nonlinear, increasing portions of the parabolic curves that were used for SRR calculations. Light gray shaded areas show zones of declining sulfide.
Diversity of sulfate-reducing populations in Mushroom Spring.
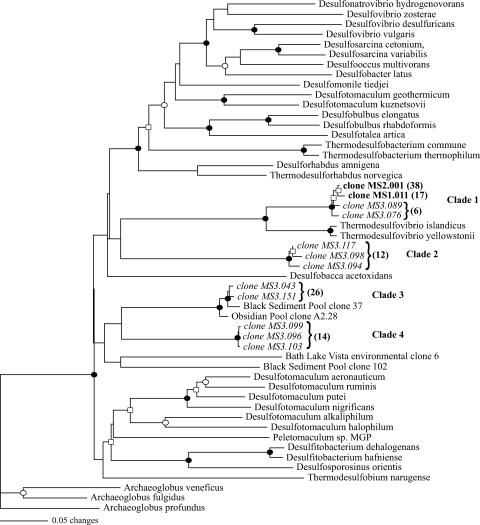
Despite screening of over 50 clones, molecular analyses revealed only a single dsrAB phylotype in the upper two millimetric layers (Fig. 5, clade 1) in direct association with the Synechococcus-Chloroflexus layers that were routinely exposed to high levels of oxygen. Surface sequences were 99% identical at the amino acid level and most closely related to the Thermodesulfovibrio clade, with amino acid identities of ca. 85% with T. yellowstonii and T. islandicus. Additionally, closely related members of this phylogenetic grouping (98% amino acid similarity) were recovered from the anoxic 15- to 18-mm layer (Fig. 5, clade 1). This clade had very strong bootstrap support (100% for each) in both neighbor joining and parsimony analyses (Fig. 5).
FIG. 5.
Unrooted dendrogram depicting relationships among dsrAB gene sequences for clones obtained from mat sections from within (0 to 1 mm and 1 to 2 mm) (in bold) and below (15 to 18 mm) (in italics) the midday oxycline and closely related sequences. The tree was constructed using an alignment of 481 amino acid positions (gaps and ambiguous residues were excluded using a filter in ARB). Symbols at branches represent nodes where bootstrap confidence values of >50% (□), >75% (○), and >90% (•) were observed for bootstrapped neighbor-joining and parsimony trees and expressed as percentages of 1,000 replicates for both. Nodes without values were unsupported by the majority of tree reconstructions in one or both methods. The total number of replicate clones in each clade or subclade in each of the clone libraries is shown in parentheses.
A greater diversity of dsrAB sequence types was detected in the anoxic layer, as three other clades were detected in the 15- to 18-mm sample (Fig. 5, clades 2 to 4). None of these three clades was closely related to any cultured SRP. One group of Mushroom Spring dsrAB sequences (Fig. 5, clade 3) had well-supported phylogenetic affiliation (100% of bootstrap replicates with both phylogenetic approaches) with sequences recovered by members of our group during a previous survey of hot springs in YNP where sulfate reduction was detected (16). The other two groups of sequences were not significantly similar to any sequences in the GenBank database (BLAST E values of >e−39 for both).
DISCUSSION
Three decades of study of laminated mats in low-sulfate thermal springs have provided a better understanding of the microbial diversity of these communities and highlighted the importance of a number of metabolic processes, including oxygenic photosynthesis by the unicellular cyanobacterium Synechococcus lividus (31), cross-feeding between cyanobacteria and photoheterotrophic Chloroflexus-type filaments (2), nitrogen fixation (34), methanogenesis (40, 47), and acetogenesis, and the importance of anaerobic processes in decomposition and mineralization (1, 12, 34). Although the contribution of sulfate-reducing bacteria to processes in photosynthetic mats in high-sulfate hypersaline environments is well documented (11, 24, 25, 29, 30, 32), their contributions to processes in mats that develop in low-sulfate environments are less well studied. However, the study of sulfate respiration in microbial mats in low-sulfate environments is especially relevant to understanding the evolution of these communities, since isotopic data indicate that sulfate levels, even in marine environments, were likely to have been <200 μM on the Archean Earth (20).
The presence of sulfate-respiring microorganisms in mats that develop in low-sulfate geothermal outflows in YNP was first shown by chemical analyses (13), demonstrating that over a 48-h period sulfide formation was associated with declining sulfate concentrations with depth. Two additional reports, using radiotracers to survey the distribution of sulfate reduction, confirmed activity in Octopus Spring (48) and Mushroom Spring (33) mats, showing rates comparable to our daytime values (91 to 483 nmol ml−1 day−1). Our analyses, examining depth-specific and depth-integrated changes in SRR over a diel period, now provide a more complete accounting of this process and its association with the populations likely contributing to the observed activities.
Although sulfate reduction was measurable at all depth intervals examined, the highest rates near the surface were observed shortly after the collapse of the oxygen chemocline at dusk or in immediate response to shading. This pattern differs from that observed in mats that develop in hypersaline habitats, where elevated daytime SRRs (7, 18, 36) have been attributed to increased temperature (7) and photosynthetic productivity (5, 8, 18). The SRR patterns in Mushroom Spring were more similar to the nightly increase in nitrogen fixation, another oxygen-sensitive process, observed in coastal microbial mat populations (3). Despite these differences from hypersaline mats, the Mushroom Spring data may also reflect an influence of irradiance-driven photosynthetic productivity on sulfate reduction. Previous studies of hypersaline mats have shown that supplementation with photoexcretion products such as glycolate stimulate sulfate respiration activity (18). Chloroflexus filaments in Mushroom Spring have been reported to consume glycolate excreted by Synechococcus (2). The dramatic increase in specific SRRs at the Mushroom Spring mat surface may simply be related to the decrease of oxygen that was inhibiting sulfate respiration or reoxidizing sulfide (see below) during the day; however, the subsequent decline of depth-integrated rates from the evening to a minimum at dawn may be due to the gradual depletion of photosynthetically derived carbon in the mat. The connection between SRR and photosynthetic productivity may also have contributed to the up-to-fourfold decrease in depth-integrated SRRs between the July 2001 and September 2003 sampling dates, perhaps reflecting seasonal changes in day length and peak irradiance between those two study periods. This is supported by microelectrode profile data that showed peak photosynthetic oxygen levels near 900 μM in September compared to levels around 1,400 μM in 2001. Although daytime SRRs in both 2001 and 2003 were comparable, it may be that the size of the evening SRR peak is related instead to the length of the day and the potentially larger accumulated pool of carbon formed over the whole day.
Our studies also point to the possible presence of an active sulfur cycle near the surface of the mat during daytime. Although appreciable sulfate reduction at the surface was not observed following short 5-minute incubations in the light, the almost instantaneous 20-fold increases in rates in response to shading observed in the 2000 dark control experiment is suggestive of an active sulfur cycle in this region. Reoxidation of sulfide, via either aerobic oxidation or anoxic photosynthesis, is inhibited when both oxygenic and anoxygenic photosynthesis is attenuated by shading (31). These data point to the existence of a resident population of SRP active near the surface, possibly respiring sulfate in the presence of oxygen or, alternatively, rapidly initiating sulfate respiration when the oxygen level drops following attenuation of light. Thus, our measurements may underestimate true daytime SRRs at the surface.
Overall, the SRRs that we detected in the current study are comparable to rates detected in a cyanobacterial mat in a high-sulfate hot spring (Bath Lake; 6 to 8 mM) in Mammoth Basin, YNP (44). Notably, the high evening-specific SRRs that we measured in 2001 (ca. 14 μmol cm−3 day−1) are comparable to the highest rates of sulfate reduction reported by Habicht and Canfield (19). In that study, SRRs of 23 to 37 μmol cm−3 day−1 were detected in microbial mat and sulfuretum sediment samples incubated in bags under artificially elevated temperatures (30°C). The high in situ rates in Mushroom Spring may be partly due to enhanced reaction rates at high temperatures (56 to 58°C), although past reports have shown similar optimal specific SRRs for cultures adapted to mesophilic or thermophilic temperatures (6).
The radiometric SRR measurements confirmed the sulfide profile data set. Past authors have attempted to measure sulfide in high-temperature springs but have encountered difficulty in calibrating voltametric electrodes (31). This report, using Clark-style amperometric sensors, represents the first measurement of sulfide in hot springs. Our results were quite typical compared with profiles collected from sediments and an aerobic biofilm (26, 27), except that the decrease in sulfide with depth that we saw does not typically occur in higher-sulfate environments. Calculated specific SRRs based on the measured sulfide profiles were very consistent with radiometric determinations of specific rates, indicating that sulfide profiles may appropriately be used to estimate SRRs in high-temperature microbial mats, although further testing of this technique should be performed.
The microelectrode profiles and radiometric data both showed similar patterns of declining sulfide production in the lower portion of the mat. This vertical pattern of declining SRRs has been repeatedly observed in marine sedimentary habitats (14, 23, 46). Those studies have typically suggested that carbon limitation is responsible for the decline of activity with depth. Because of the vanishingly low [SO4] in Mushroom Spring, it may be a combined effect of carbon and/or sulfate limitation that is responsible for the decline in this ecosystem.
Analysis of dsrAB sequence types revealed four major clades of sulfate-reducing phylotypes that were differentially distributed with depth in the mat. Three were recovered only from the exclusively anoxic zone of the mat, consistent with a traditional understanding of the habitat preference of SRP. One of these groups (clade 3) showed significant homology with sequences isolated from other thermal habitats in YNP. Interestingly, temperatures among these sites vary and neither Obsidian Pool (89°C; pH 6.9; [SO4], 0.54 mM) nor Black Sediment Pool (69°C; pH 6.6; [SO4], 0.6 mM), where those related sequences were obtained, contain photosynthetic microbial mats; however, like Mushroom Spring, they both have relatively low sulfate concentrations. This suggests that this dsrAB clade may be broadly distributed among low-sulfate thermal habitats.
In contrast, Thermodesulfovibrio-like dsrAB genotypes were recovered from both oxic and anoxic depths. Although SRP in Octopus Spring have been reported (43), including a Deltaproteobacterium- and a Thermodesulfobacterium-like sequence, this is the first observation of a Thermodesulfovibrio-like population in a hot spring microbial mat. A significant contribution of these Thermodesulfovibrio-like species to sulfate reduction was also supported by the recovery of this sequence type in total bacterial 16S rRNA gene clone libraries (2 to 4% of clones) generated from DNA extracted from the surface of the mat (unpublished observations). Thus, this possibly more oxygen-tolerant population is likely responsible for the immediate production of sulfide observed near the surface following shading of the mat.
Variable depth distributions of different SRP phylotypes have also been observed in other stratified systems, including microbial mats (29, 32). A trend observed in both our study and one of those hypersaline mat studies was that some dsrAB populations were uniformly distributed with depth, while others were more localized (29). However, analyses of dsrAB phylotypes in the hypersaline mats of Solar Lake (Sinai, Egypt) and Exportadora de Sal Saltworks (Guerrero Negro, Mexico) (30, 32; J. G. Dillon, S. Miller, B. Bebout, N. Pinel, and D. A. Stahl., unpublished data) have revealed a diversity of sequence types significantly greater than that in the samples recovered from Mushroom Spring, consistent with the view that the conditions in hot springs restrict the overall diversity of biological communities (4, 43). In particular, the low sulfate availability may specifically restrict the diversity of SRP in this spring.
Our findings demonstrate the importance of sulfate respiration as a terminal process in thermophilic microbial mats, despite fluctuating oxygen levels and very low [SO4] values. Dramatic changes in sulfate reduction between day and night, especially during the evening transition, emphasize the dynamic nature of photosynthetic microbial mats and the limitations of basing conclusions on single sampling points. Other studies of sulfate-rich saline habitats have detected significant SRRs even within the hyperoxygenated surface layers of photosynthetic mats during daytime (7). Although we observed a depression of rates during daytime, as was true in that study, we have detected some of the highest in situ sulfate respiration rates ever reported. These results, along with those of recent studies of pure cultures (10), have challenged the traditional view that all SRP are obligate anaerobes and suggest that further work for improving the understanding of the complex biogeochemical interplay of photosynthesis and sulfate respiration within the context of microbial mats is needed. This should include work investigating the potential transfer of photosynthetic carbon to SRP over the diel period. Future studies, including targeted efforts (cultivation based and cultivation independent) to study potentially oxygen-tolerant SRP, such as the Thermodesulfovibrio-like group identified in this study, are also needed to confirm the role of this group in the Mushroom Spring mat. More generally, an expanded study of the role of sulfate respiration in other low-sulfate thermal habitats, both with and without microbial mats, is warranted to determine the overall importance of the sulfur cycle in these environments.
Acknowledgments
This work was supported by NSF-IGERT grant (DGE-9870713) funding to J.G.D. and NSF grant (DEB-0213186) and NASA NAI grant (NCC2-1273) support to D.A.S. and J.G.D.
We thank the National Park Service for granting permission to perform research in the hot spring under permit number YELL 19994098. We thank Mary Bateson and David Ward for technical assistance and the use of their laboratory facility and Stahl laboratory members José de la Torre, Nicolás Pinel, Martin Könneke, and Christopher Walker for assistance in the field.
Footnotes
Published ahead of print on 15 June 2007.
REFERENCES
- 1.Anderson, K. L., T. A. Tayne, and D. M. Ward. 1987. Formation and fate of fermentation products in hot spring cyanobacterial mats. Appl. Environ. Microbiol. 53:2343-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateson, M. M., and D. M. Ward. 1988. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl. Environ. Microbiol. 54:1738-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebout, B. M., H. W. Paerl, K. M. Crocker, and L. E. Prufert. 1987. Diel interactions of oxygenic photosynthesis and N2 fixation (acetylene reduction) in a marine microbial mat community. Appl. Environ. Microbiol. 53:2353-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock, T. D. 1978. Thermophilic microorganisms and life at high temperatures. Springer-Verlag, New York, NY.
- 5.Canfield, D. E., and D. J. Des Marais. 1993. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim. Cosmochim. Acta 57:3971-3984. [DOI] [PubMed] [Google Scholar]
- 6.Canfield, D. E., K. S. Habicht, and B. Thamdrup. 2000. The Archean sulfur cycle and the early history of atmospheric oxygen. Science 288:658-661. [DOI] [PubMed] [Google Scholar]
- 7.Canfield, D. E., and D. J. D. Marais. 1991. Aerobic sulfate reduction in microbial mats. Science 251:1471-1473. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, Y. 1984. Oxygenic photosynthesis, anoxygenic photosynthesis and sulfate reduction in cyanobacterial mats, p. 435-441. In M. J. Klug and C. A. Reddy (ed.), Current perspectives in microbial ecology. American Society for Microbiology, Washington, DC.
- 9.Cohen, Y., and E. Rosenberg. 1989. Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, DC.
- 10.Cypionka, H. 2000. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 54:827-848. [DOI] [PubMed] [Google Scholar]
- 11.Des Marais, D. J. 2003. Biogeochemistry of hypersaline microbial mats illustrates the dynamics of modern microbial ecosystems and the early evolution of the biosphere. Biol. Bull. 204:160-167. [DOI] [PubMed] [Google Scholar]
- 12.Doemel, W. N., and T. D. Brock. 1977. Structure, growth, and decomposition of laminated algal-bacterial mats in alkaline hot springs. Appl. Environ. Microbiol. 34:433-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doemel, W. N., and T. D. Brock. 1976. Vertical distribution of sulfur species in benthic algal mats. Limnol. Oceanogr. 21:237-244. [Google Scholar]
- 14.Ferdelman, T. G., H. Fossing, K. Neumann, and H. D. Schulz. 1999. Sulfate reduction in surface sediments of the southeast Atlantic continental margin between 15 degrees 38′S and 27 degrees 57′S (Angola and Namibia). Limnol. Oceanogr. 44:650-661. [Google Scholar]
- 15.Ferris, M. J. 2003. Microbially mediated sulphide production in a thermal, acidic algal mat community in Yellowstone National Park. Environ. Microbiol. 5:954-960. [DOI] [PubMed] [Google Scholar]
- 16.Fishbain, S., J. G. Dillon, H. L. Gough, and D. A. Stahl. 2003. Linkage of high rates of sulfate reduction in Yellowstone hot springs to unique sequence types in the dissimilatory sulfate respiration pathway. Appl. Environ. Microbiol. 69:3663-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fossing, H., and B. B. Jørgensen. 1989. Measurement of bacterial sulfate reduction in sediments: evaluation of a single step chromium reduction method. Biogeochemistry 8:205-222. [Google Scholar]
- 18.Fründ, C., and Y. Cohen. 1992. Diurnal cycles of sulfate reduction under oxic conditions in cyanobacterial mats. Appl. Environ. Microbiol. 58:70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habicht, K. S., and D. E. Canfield. 1997. Sulfur isotope fractionation during bacterial sulfate reduction in organic-rich sediments. Geochim. Cosmochim. Acta 61:5351. [DOI] [PubMed] [Google Scholar]
- 20.Habicht, K. S., M. Gade, B. Thamdrup, P. Berg, and D. E. Canfield. 2002. Calibration of sulfate levels in the Archean Ocean. Science 298:2372-2374. [DOI] [PubMed] [Google Scholar]
- 21.Holmer, M., and P. Storkholm. 2001. Sulphate reduction and sulphur cycling in lake sediments: a review. Freshw. Biol. 46:431-451. [Google Scholar]
- 22.Javor, B. 1989. Hypersaline environments: microbiology and biogeochemistry. Springer-Verlag, New York, NY.
- 23.Jørgensen, B. B. 1982. Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Philos. Trans. R. Soc. Lond. B 298:543-561. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen, B. B. 1977. Solar Lake (Sinai). 5. The sulfur cycle of the benthic cyanobacterial mats. Limnol. Oceanogr. 22:657-666. [Google Scholar]
- 25.Krekeler, D., P. Sigalevich, A. Teske, H. Cypionka, and Y. Cohen. 1997. A sulfate-reducing bacterium from the oxic layer of a microbial mat from Solar Lake (Sinai), Desulfovibrio oxyclinae sp. nov. Arch. Microbiol. 167:369-375. [Google Scholar]
- 26.Kühl, M., and B. B. Jørgensen. 1992. Microsensor measurements of sulfate reduction and sulfide oxidation in compact microbial communities of aerobic biofilms. Appl. Environ. Microbiol. 58:1164-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kühl, M., C. Steuckart, G. Eickert, and P. Jeroschewski. 1998. A H2S microsensor for profiling biofilms and sediments: application in an acidic lake sediment. Aquat. Microb. Ecol. 15:201-209. [Google Scholar]
- 28.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüssmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K.-H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minz, D., S. Fishbain, S. J. Green, G. Muyzer, Y. Cohen, B. E. Rittmann, and D. A. Stahl. 1999. Unexpected population distribution in a microbial mat community: sulfate-reducing bacteria localized to the highly oxic chemocline in contrast to a eukaryotic preference for anoxia. Appl. Environ. Microbiol. 65:4659-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minz, D., J. L. Flax, S. J. Green, G. Muyzer, Y. Cohen, M. Wagner, B. E. Rittmann, and D. A. Stahl. 1999. Diversity of sulfate-reducing bacteria in oxic and anoxic regions of a microbial mat characterized by comparative analysis of dissimilatory sulfite reductase genes. Appl. Environ. Microbiol. 65:4666-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Revsbech, N. P., and D. M. Ward. 1984. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl. Environ. Microbiol. 48:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risatti, J. B., W. C. Chapman, and D. A. Stahl. 1994. Community structure of a microbial mat: the phylogenetic dimension. Proc. Natl. Acad. Sci. USA 91:10173-10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roychoudhury, A. N. 2004. Sulfate respiration in extreme environments: a kinetic study. Geomicrobiol. J. 21:33-43. [Google Scholar]
- 34.Steunou, A. S., D. Bhaya, M. M. Bateson, M. C. Melendrez, D. M. Ward, E. Brecht, J. W. Peters, M. Kühl, and A. R. Grossman. 2006. In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc. Natl. Acad. Sci. USA 103:2398-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamimi, A., E. B. Rinker, and O. C. Sandall. 1994. Diffusion coefficients for hydrogen sulfide, carbon dioxide, and nitrous oxide in water over the temperature range 293-368 K. J. Chem. Eng. Data 39:330-332. [Google Scholar]
- 36.Teske, A., N. B. Ramsing, K. Habicht, M. Fukui, J. Kåver, B. B. Jørgensen, and Y. Cohen. 1998. Sulfate-reducing bacteria and their activities in cyanobacterial mats of Solar Lake (Sinai, Egypt). Appl. Environ. Microbiol. 64:2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich, G., L. Krumholz, and J. Suflita. 1997. A rapid and simple method for estimating sulfate reduction activity and quantifying inorganic sulfides. Appl. Environ. Microbiol. 63:1627-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent, W. F., and C. Howard-Williams. 1986. Antarctic stream ecosystems: physiological ecology of a blue-green algal epilithon. Freshw. Biol. 16:219-233. [Google Scholar]
- 39.Wagner, M., A. J. Roger, J. L. Flax, G. A. Brusseau, and D. A. Stahl. 1998. Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J. Bacteriol. 180:2975-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, D. M. 1978. Thermophilic methanogenesis in a hot-spring algal-bacterial mat (71 to 30°C). Appl. Environ. Microbiol. 35:1019-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward, D. M., E. Beck, N. P. Revsbech, K. A. Sandbeck, and M. R. Winfrey. 1984. Decomposition of hot spring microbial mats, p. 191-214. In Y. Cohen, R. W. Castenholz, and H. O. Halvorson (ed.), Microbial mats: stromatolites, vol. 3. Alan R. Liss, Inc., New York, NY. [Google Scholar]
- 42.Ward, D. M., and R. W. Castenholz. 2000. Cyanobacteria in geothermal habitats, p. 37-59. In B. A. Whitton and M. Potts (ed.), The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 43.Ward, D. M., M. J. Ferris, S. C. Nold, and M. M. Bateson. 1998. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 62:1353-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward, D. M., and G. J. Olson. 1980. Terminal processes in the anaerobic degradation of an algal-bacterial mat in a high-sulfate hot spring. Appl. Environ. Microbiol. 40:67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward, D. M., R. Weller, J. Shiea, R. Castenholz, and Y. Cohen. 1989. Hot spring microbial mats: anoxygenic and oxygenic mats of possible evolutionary significance, p. 3-15. In Y. Cohen and E. Rosenberg (ed.), Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, DC.
- 46.Weber, A., W. Reiss, F. Wenzhoefer, and B. B. Jorgensen. 2001. Sulfate reduction in Black Sea sediments: in situ and laboratory radiotracer measurements from the shelf to 2000 m depth. Deep Sea Res. A 48:2073-2096. [Google Scholar]
- 47.Zeikus, J. G., A. Ben-Bassat, and P. W. Hegge. 1980. Microbiology of methanogenesis in thermal, volcanic environments. J. Bacteriol. 143:432-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeikus, J. G., M. A. Dawson, T. E. Thompson, K. Ingvorsen, and E. C. Hatchikian. 1983. Microbial ecology of volcanic sulfidogenesis. Isolation and characterization of Thermodesulfobacterium commune gen. nov. and sp. nov. J. Gen. Microbiol. 129:1159-1169. [Google Scholar]