Abstract
Human β-hexosaminidase A (HexA) is a heterodimeric glycoprotein composed of α- and β-subunits that degrades GM2 gangliosides in lysosomes. GM2 gangliosidosis is a lysosomal storage disease in which an inherited deficiency of HexA causes the accumulation of GM2 gangliosides. In order to prepare a large amount of HexA for a treatment based on enzyme replacement therapy (ERT), recombinant HexA was produced in the methylotrophic yeast Ogataea minuta instead of in mammalian cells, which are commonly used to produce recombinant enzymes for ERT. The problem of antigenicity due to differences in N-glycan structures between mammalian and yeast glycoproteins was potentially resolved by using α-1,6-mannosyltransferase-deficient (och1Δ) yeast as the host. Genes encoding the α- and β-subunits of HexA were integrated into the yeast cell, and the heterodimer was expressed together with its isozymes HexS (αα) and HexB (ββ). A total of 57 mg of β-hexosaminidase isozymes, of which 13 mg was HexA (αβ), was produced per liter of medium. HexA was purified with immobilized metal affinity column for the His tag attached to the β-subunit. The purified HexA was treated with α-mannosidase to expose mannose-6-phosphate (M6P) residues on the N-glycans. The specific activities of HexA and M6P-exposed HexA (M6PHexA) for the artificial substrate 4MU-GlcNAc were 1.2 ± 0.1 and 1.7 ± 0.3 mmol/h/mg, respectively. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis pattern suggested a C-terminal truncation in the β-subunit of the recombinant protein. M6PHexA was incorporated dose dependently into GM2 gangliosidosis patient-derived fibroblasts via M6P receptors on the cell surface, and degradation of accumulated GM2 ganglioside was observed.
A human lysosomal enzyme, β-hexosaminidase A (HexA; EC 3.2.1.52), hydrolyzes β-glycosidically linked N-acetylglucosamine or N-acetylgalactosamine residues at the nonreducing ends of glycoconjugates. With the coactivation of GM2 activator protein, HexA degrades GM2 gangliosides in the lysosome (27). HexA is composed of two subunits, α and β, with 57% similarity in their amino acid sequences. Normally, mammalian cells contain three isozymes, HexA (αβ heterodimer), HexS (αα homodimer), and HexB (ββ homodimer). HexA and HexB are the major forms, and HexS is a labile minor form (13, 32). Unlike HexA, the two homodimers are incapable of hydrolyzing GM2 ganglioside; however, there are artificial substrates that are hydrolyzed by all three isozymes. Deficient HexA activity leads to a lysosomal storage disease known as GM2 gangliosidosis, which causes neuronal pathologies. There are three types of GM2 gangliosidosis: Tay-Sachs disease (TS), Sandhoff disease (SD), and AB variant, with defects in the α-subunit, β-subunit, and GM2 activator protein, respectively.
For the treatment of lysosomal storage diseases (including GM2 gangliosidosis), chemical chaperones (3, 42), substrate deprivation (2, 5), enzyme replacement (4, 9, 38, 46), gene therapy (1, 17, 20, 39), and bone marrow transplantation (14, 29) have been attempted. Of these methods, enzyme replacement therapy (ERT) is a strategy that has been clinically approved for Gaucher disease (4), Fabry disease (9, 38), Pompe disease (18, 45), and mucopolysaccharidosis I (46), II (28), and VI (12). Because ERT provides fully active recombinant enzymes that are produced in mammalian cells, it is suitable for most patients with or without expression of the enzyme responsible for the disease, and no complicated surgery is required. The administered recombinant enzymes harbor mannose or mannose-6-phosphate (M6P) residues in N-glycans; these residues are recognized by the respective receptors on the cell surface and incorporated into the cell.
At present, the recombinant enzymes prescribed for ERT have been produced in mammalian cells because the glycan structure is similar to that of native enzyme. This similarity is preferable because of the necessary M6P exposure and the antigenicity that could be caused by the heterogenic glycan structure of recombinant enzymes. However, it is expensive to produce adequate amounts of protein for therapeutic purposes from mammalian cells. Chiba et al. (8) produced recombinant α-galactosidase A (α-GalA) in a modified yeast strain; they manipulated Saccharomyces cerevisiae to synthesize glycoprotein that lacks the outer chain of N-glycan, a structure specific to yeast but not to humans. The purified recombinant α-GalA was effectively introduced into Fabry patient fibroblasts and a Fabry mouse model and successfully hydrolyzed accumulating substrates (8, 37). These results support the possibility of using yeast as a host to produce recombinant enzymes for ERT.
Because HexA is the only one of the three isozymes that hydrolyzes GM2 ganglioside, administration of HexA is indispensable for ERT of TS and SD. The replacement of recombinant β-hexosaminidases in CHO cells applied to SD mouse microglia, Schwann cells, and SD human fibroblasts has been reported (33, 44). To further investigate the use of ERT for GM2 gangliosidosis, in this report we have produced yeast recombinant HexA that can be incorporated into TS and SD cells and hydrolyzes accumulating intracellular GM2 gangliosides. While α-GalA was produced in S. cerevisiae (8), the expression of HexA was performed in the methylotrophic yeast Ogataea minuta. We chose O. minuta as a host for HexA expression for two reasons: the methylotrophic yeast is more suited for massive production of recombinant enzymes than S. cerevisiae, as suggested by greater α-GalA expression in Pichia pastoris (7) than in S. cerevisiae (8), and the och1-disrupted strain of O. minuta (TK5-3) produces glycoprotein without an outer chain specific to yeast glycoprotein (19), which potentially solves the problem of antigenic glycans. Recombinant HexA was successfully expressed as a heterodimer of α- and β-subunits and examined for its ability to be utilized for ERT of TS and SD.
MATERIALS AND METHODS
Materials.
The methylotrophic yeasts Ogataea minuta strain TK5-3 (och1Δ ura3Δ ade1Δ), kindly provided by Kirin Brewery Co., Ltd. (19), and Pichia pastoris strain GS115 (his4Δ) (Invitrogen, Carlsbad, CA) were used to express recombinant human β-hexosaminidase and M6P receptor domain 9 (Dom9His), respectively. Cultured skin fibroblasts from a patient with SD and a healthy subject were established and maintained in our laboratory (33). TS WG1051 fibroblasts (21) were obtained from the Repository for Mutant Cell Strains (Montreal, Canada). Anti-human HexA antibody was prepared from rabbit (16, 43). The following materials were purchased from commercial sources: human placenta β-hexosaminidase, Sigma-Aldrich (St. Louis, MO); chromatography medium for purification, GE Healthcare Bio-Sciences Corp. (Piscataway, NJ); medium for fibroblasts, Gibco (Grand Island, NY); broth for bacteria and yeast strains, Becton Dickinson and Co. (Franklin Lakes, NJ); and jar fermentor and process control system, EYELA (Tokyo Rikakikai Co., Tokyo, Japan).
Construction of expression plasmids.
The α- and β-subunit genes, HEXA (accession number NM_000520) and HEXB (accession number M19735), were amplified by PCR from cDNA provided by R. L. Proia (NIH). Amplified HEXA and HEXB fragments were inserted into expression vectors for O. minuta with markers URA3 (19) and ADE1, respectively. The constructed plasmids pOMEU1-HEXA, amplified by sense primer (5′-CGAAAAAATCTAGAATGACAAGC-3′) and antisense primer (5′-AAGGATCCTCAGGTCTGTTCAAACTCCTGCTCAC-3′), and pOMEA1-HEXB, amplified by sense primer (5′-ATTCTAGAAAAATGCTGCTGGCGCTGCTGT-3′) and antisense primer (5′-CGCTCTAGATTACATGTTCTCATGGT-3′), were designed to express the α- and β-subunits of HexA, respectively, with their own secretion signal sequences. The fragment coding for only mature β-subunit (HEXB) from Ala92 to Met600 (with numbering according to the sequences shown below in Fig. 2C) was also amplified by PCR with a sense primer (5′-TAATCTAGACCCGGGCCAAGCCG-3′) and antisense primer (5′-CGCTCTAGATTACATGTTCTCATGGT-3′), and the fragment was first inserted to the XbaI site of pUC19. After confirmation of the sequence, the SmaI and HincII fragment was inserted into the SrfI site of pOMEA1-His6, an expression vector for O. minuta with the ADE1 marker (pOMEA1-HisHEXB). This plasmid was designed to express mature β-subunit with α-factor pre-pro and the His tag sequences attached to its N terminus as a secretion signal and an affinity tag for purification. Recombinant Dom9His was produced from P. pastoris GS115 as described by Hancock et al. (11), except that the human kidney cDNA library (Marathon-Ready cDNA; Clontech, Mountain View, CA) and pPIC9 (Invitrogen) were used as the template and expression vector, respectively.
FIG. 2.
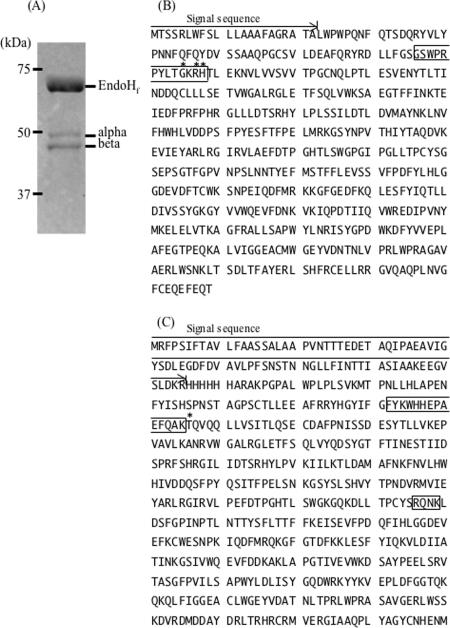
Primary structure and inner processing sites of the α- and β-subunits of human HexA. (A) EndoHf-treated HexA blotted onto PVDF membrane and stained with CBB. The EndoHf treatment was performed according to the manufacturer's instructions. The two major bands (α and β) were analyzed for their N-terminal sequences. The primary structures of the α-subunit (B) and the β-subunit (C) of HexA are shown. Asterisks show the N-terminal amino acid detected by protein sequencing. Signal sequences are shown by the arrows. Amino acids that are proteolytically processed in mammalian HexA are boxed.
Transformation of methylotrophic yeast.
O. minuta TK5-3 was transformed with NotI-digested pOMEU1-HEXA and pOMEA1-HEXB for the expression of recombinant HexA without the His tag. For the expression of His-tagged HexA, pOMEA1-HisHEXB was used instead of pOMEA1-HEXB. The O. minuta cells used for transformation were prepared as described by Kuroda et al. (19). The transformation of P. pastoris for expression of Dom9His was performed according to the manufacturer's instructions.
Expression and purification of recombinant β-hexosaminidase isozymes and Dom9His.
The transformed O. minuta was precultured in 100 ml of YPAD broth (2% peptone, 1% yeast extract, 2% glucose, and 0.2 mg/ml adenine) and then transferred to 6 liters of BMGY broth (6% peptone, 1% yeast extract, 1.34% yeast nitrogen base without amino acids, 1% glycerol, and 0.1 M potassium phosphate [pH 6.0]) in a jar fermentor. When the glycerol was completely consumed, methanol was added as a carbon source and inducer. Methanol induction was performed at 26 to 28°C and continued until the 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide (MUG; Sigma Aldrich)-hydrolyzing activity in the culture broth reached saturation. The temperature and dissolved oxygen concentration were monitored and controlled by a computer during fermentation. After the induction, the supernatant of the cultured medium was concentrated by ultrafiltration (Microza UF; Asahi Kasei Chemicals Corp., Tokyo, Japan) and collected as a crude enzyme.
The crude enzyme without His tag was partially purified with a HiTrap butyl column (GE Healthcare Bio-Sciences Corp.) followed by a HiTrap DEAE column (GE Healthcare Bio-Sciences Corp.). DEAE chromatography was performed to separate isozymes by a NaCl gradient (0 to 300 mM) as previously described by Tsuji et al. (44), with a slight modification in the program of elution of isozymes. Three fractions with MUG-hydrolyzing activity were separately collected as recombinant HexB (ββ), HexA (αβ), and HexS (αα); these fractions were eluted at NaCl concentrations of 50 mM, 90 to 150 mM, and 190 to 250 mM, respectively.
To purify His-tagged HexA, the crude enzyme that contained HexA (heterodimer of α- and His-tagged β-subunits), HexS (homodimer of α-subunits), and a trace amount of HexB (homodimer of His-tagged β-subunits) was adjusted to pH 7.2 and applied to a HisTrap column (GE Healthcare Bio-Sciences Corp.), an affinity column for His-tagged protein. His-tagged HexA was eluted with 100 mM imidazole in sodium phosphate buffer (pH 7.2) containing 500 mM NaCl, and the elution was desalted and concentrated. The use of a yeast-derived enzyme in ERT requires α-mannosidase treatment to expose M6P residues, because the M6P in yeast N-glycans is covered by mannose residues and positioned a few residues inside from the nonreducing end. The α-mannosidase treatment of purified enzyme was performed as described previously (8), and the HexA was recollected by using a HisTrap column. The purified enzyme was named M6PHexA.
Dom9His was expressed and purified according to the method described by Reddy et al. (36). All chromatograms were performed at 4°C under the recommended conditions for the respective columns.
Enzyme assays.
β-Hexosaminidase activity was assayed with MUG (Sigma-Aldrich), 4-methylumbelliferyl-6-sulfo-N-acetyl-β-d-glucosaminide (MUGS; Calbiochem, San Diego, CA), and 4-nitrophenyl N-acetyl-β-d-glucosaminide (pNP-GlcNAc; Sigma-Aldrich) as substrates (34, 41). Enzyme activity was determined as the amount of 4-methylumbelliferone or 4-nitrophenol liberated in 1 h at 37°C per mg of protein.
To detect GM2 ganglioside degradation, the following reaction mixture was prepared in a total volume of 200 μl: 8 μg of GM2 from bovine brain (Sigma-Aldrich), 20 μg of bovine serum albumin, 200 μg of sodium taurodeoxycholate, and 300 nmol/h (toward MUGS) of HexA or human placenta β-hexosaminidase (Sigma-Aldrich) in 20 mM McIlvaine buffer (pH 4.2). This reaction mixture was incubated at 37°C for 1, 3, and 24 h. The reaction was terminated by boiling, desalted with a Sep-Pak column (Waters Corp., Milford, MA), dried in a vacuum, and redissolved in 50 μl of methanol. The methanol suspension was used in thin-layer chromatography (TLC) performed by a solvent system of chloroform-methanol-0.2% CaCl2 (60/35/8, vol/vol/vol). Then, resorcinol reagent (2 mg/ml resorcinol, 80% HCl, and 0.25 mM CuSO4) was sprayed onto the plate and heated at 115°C for 30 min.
The amount of protein was measured using the BCA protein assay reagent kit (Pierce, Rockford, IL). The α-mannosidase activity was measured as described previously (8).
N-terminal analysis of recombinant HexA.
Purified HexA was treated with endoglycosidase H (EndoHf; New England BioLabs, Ipswich, MA) according to the manufacturer's instructions. It was then used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto polyvinylidene difluoride (PVDF) membrane, and stained with Coomassie brilliant blue (CBB). The two SDS-PAGE bands were analyzed by Edman sequencing using the Procise 494 HT protein sequencing system (Applied Biosystems, Foster City, CA).
Preparation of antibodies against HEX α- and β-subunits.
Polyclonal antibodies for detecting HEX α- and β-subunits were prepared in rabbits by using two keyhole limpet hemocyanin-conjugated synthetic peptides that corresponded to amino acids 277 to 289 of the α-subunit (CYSGSEPSGTFGP) and amino acids 556 to 571 of the β-subunit (C-RLWSSKDVRDMDDAYD) as antigens (numbering according to Fig. 2B and C, below). The antibodies recognized both the mature and precursor forms of the proteins on immunoblotting.
Detection of M6P exposed on N-glycans.
Interaction between the recombinant enzyme and the M6P receptor was detected by receptor staining with Dom9His produced from Pichia pastoris. A pair of blotted PVDF membranes was prepared from HexA and M6PHexA that was subjected to SDS-PAGE. One membrane was used to perform immunostaining with anti-human HexA serum, and the other membrane was used to detect molecules interacting with Dom9His in combination with the PentaHis-horseradish peroxidase conjugate kit (QIAGEN, Hilden, Germany). Briefly, after the membrane was blocked, it was incubated with Dom9His, followed by anti-His antibody conjugated with horseradish peroxidase solution (QIAGEN). The Dom9His bound to M6PHexA was detected with the ECL plus Western blotting detection system (GE Healthcare Bio-Sciences Corp.). The detection patterns of both membranes were compared.
N-glycan analysis.
The percentage of N-glycan that contained M6P was determined by high-performance liquid chromatography (HPLC) analysis of pyridylamine (PA)-labeled N-glycans from recombinant HexA. Briefly, the N-glycans in HexA were isolated by digestion with peptide N-glycosidase F (PNGase F; New England BioLabs), followed by the removal of proteins with ethanol precipitation. The collected glycans were labeled with PA by using a Palstation pyridylamination reagent kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. The PA-labeled glycans were roughly separated into phosphorylated and nonphosphorylated fractions with a normal-phase column, Shodex Asahipak NH2P-50 4E (4.6-mm inner diameter by 25 cm; Showa Denko K.K., Tokyo, Japan). Then, the glycans were fractionated by size with the TSK-gel Amide-80 column (4.6 mm by 25 cm; Tosoh, Tokyo, Japan) as described by Takashiba et al. (40) with a slight modification to the HPLC protocol. The solvent system used for both chromatograms was 100% acetonitrile as solvent A and 200 mM triethylamine acetate (pH 7.0) in acetonitrile as solvent B. Glycan separation was performed by increasing the concentration of solvent B from 40% to 75% over a period of 60 min for the NH2P-50 column and from 30% to 62% over a period of 40 min for the Amide-80 column. Both chromatograms were performed at a flow rate of 1 ml/min at 40°C. The amount of each N-glycan was indicated by the percentage of total peak areas in the Amide-80 column elution.
Uptake of recombinant HexA and immunofluorescence analysis of intracellular GM2 ganglioside in TS and SD fibroblasts.
The cells were grown in Eagle's medium (Dulbecco's modified Eagle's medium) or Ham's F-10 medium supplemented with 10% fetal calf serum at 37°C in a humidified incubator flushed continuously with a 5% CO2-95% air mixture. Experiments for enzyme uptake by TS and SD fibroblasts and the observation of intracellular GM2 ganglioside by immunostaining against anti-GM2 antibody were performed as described previously (33, 44). Briefly, for enzyme incorporation, TS and SD fibroblasts were cultured for 3 days in medium containing HexA with a particular amount of MUGS-hydrolyzing activity (0, 200, 600, or 1,800 nmol/h/well). Every 24 h during the cell culture, half of the medium was exchanged with fresh new medium containing HexA with the same amount of MUGS-hydrolyzing activity. For the inhibition of M6P receptor-mediated uptake, 5 mM M6P was added to the medium prior to the enzyme addition. For detection of enzyme incorporation, the cell extract was assayed for MUGS-hydrolyzing activity and protein concentration, and the intracellular GM2 ganglioside degradation was examined as described previously (33, 44), except that anti-ganglioside GM2 rabbit polyclonal antibody (Calbiochem) was used as a primary antibody and a BX-FLA microscope (Olympus Corp., Tokyo) was used to observe the stained cells.
RESULTS
Expression, purification, and characterization of recombinant HexA.
Cultured broth of recombinant O. minuta was separated with a HiTrap DEAE column to estimate the ratio of isozymes produced. Compared to that of mammalian β-hexosaminidase (44, 48), the recombinant β-hexosaminidase produced in yeast displayed a different pattern on an anion exchange chromatogram (HiTrap DEAE column) (data not shown). The ratio of HexB, HexA, and HexS obtained by DEAE column chromatography was 1/11/35, suggesting that the amount of HexA produced is about 23% of the total isozymes produced and that HexB, the most abundant isozyme in mammalian cells, was barely produced in yeast.
For rapid purification of HexA with a high recovery using an immobilized metal affinity column, His-tagged HexA (His tag attached to the N terminus of the β-subunit) was prepared. The tag attached to the recombinant enzyme may create a problem with antigenicity, which would make the tagged recombinant enzyme unsuitable for ERT. However, since the experiments in this study were performed only in vitro or in situ to examine the possibility of yeast recombinant proteins for therapeutic usage, the antigenicity of the His tag was not a concern at this stage. Therefore, for further experiments in this report, His-tagged HexA was used.
His-tagged recombinant enzyme was prepared from the culture broth of HexA-expressing O. minuta in a jar fermentor and was purified as shown in Table 1. Neither HexB nor HexS was detected in the Western blotting analysis on native PAGE of purified HexA when using anti-HexA, anti-α-subunit, and anti-β-subunit antibodies (data not shown), suggesting that HexA is effectively purified by the HisTrap column. The ratio of MUG- to MUGS-hydrolyzing activities of purified HexA and M6PHexA increased to 2.6 and 2.9 from the value for crude enzyme (1.7), respectively. This value is slightly lower than the reported values (3.5 to 4.0) for CHO recombinant HexA (15, 44). The purified HexA and M6PHexA from four batches of culture were combined and used for further investigations.
TABLE 1.
Purification of recombinant HexA and M6PHexA
| Step no. | Purification method | Protein (mg) | Total activity (mmol/h)
|
Sp act (mmol/h/mg)
|
Recovery of MUG (%) | Purification (fold) of MUG | MUG/MUGS ratio | ||
|---|---|---|---|---|---|---|---|---|---|
| MUG | MUGS | MUG | MUGS | ||||||
| 1 | Culture supernatant, 4 liters | 1,500 | 74 | 43 | 0.051 | 0.029 | 100 (23)a | 1.0 | 1.7 |
| 2 | HisTrap (HexA) | 7.6 | 11 | 4.3 | 1.4 | 0.57 | 14 | 28 | 2.6 |
| 3 | Mannosidase treatment | 16 | 7.9 | 3.2 | 0.51 | 0.20 | 11 | 10 | 2.5 |
| 4 | HisTrap (M6PHexA) | 3.3 | 4.9 | 1.7 | 1.5 | 0.50 | 6.6 | 29 | 2.9 |
Amount of HexA in the total isozymes, calculated from their ratio as detected by DEAE elution. Steps 2 and 4 describe purified HexA and M6PHexA, respectively.
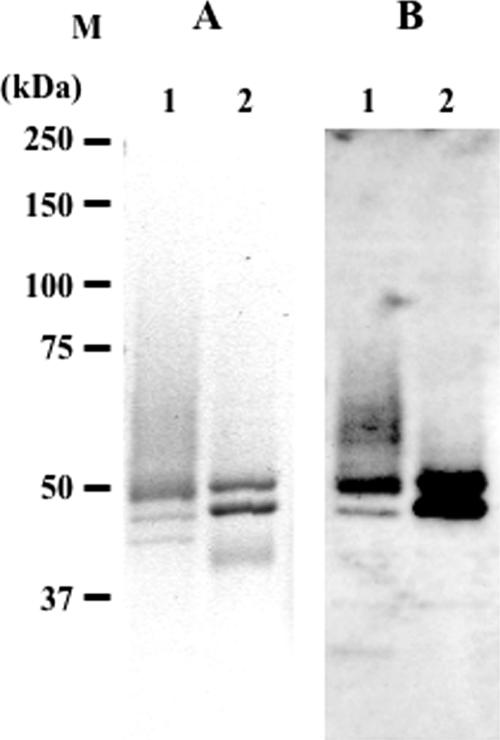
The amount of recombinant β-hexosaminidase isozymes produced from yeast cells in 1 liter of culture broth was about 57 mg, of which 23% (13 mg) was HexA, based on the ratio of isozymes separated by the DEAE column. The SDS-PAGE and immunostaining analysis of purified HexA and M6PHexA (Fig. 1) showed two major bands, probably derived from the α- and β-subunits. The smear signal observed with HexA (lanes 1) disappeared with M6PHexA (lanes 2) as a result of α-mannosidase treatment. The SDS-PAGE pattern of deglycosylated HexA by EndoHf showed two bands with apparent molecular masses of 46 kDa and 43 kDa (Fig. 2A). The N-terminal amino acid sequence of the 46-kDa band was identified by a protein sequencer as a mixture of G85KRHT, R87HTLE, and H88TLEK, and that of the 43-kDa band was identified as T166QVQQ (numbered according to the sequences in Fig. 2). The sequence of the 46-kDa band, which was determined to be the mature α-subunit, started several amino acid residues upstream of native enzyme, starting from T89 (Fig. 2B). The sequence of the 43-kDa band, which was determined to be the β-subunit, was processed at a single site starting from T166 (Fig. 2C), as reported for human HexA (24, 35). Based on the size estimated from SDS-PAGE, the second processing site of the β-subunit (R356QNK) (Fig. 2C) was not processed in the O. minuta recombinant protein. From the primary structure of the β-hexosaminidase subunits, the molecular mass of the processed α-subunit from Gly85, Arg87, or His88 to the C terminus (Thr) is 50.7 to 51.0 kDa and that of the β-subunit is 49.9 kDa (Thr166 to Met). Differences in the molecular mass of a protein often occur between the molecular mass deduced from SDS-PAGE and that calculated from the primary structure, and a similar result was observed for the α- and β-subunits of HexA. The C terminus of the β-subunit, however, appears to be truncated in O. minuta recombinant HexA, as described in the Discussion section, below.
FIG. 1.
Analysis of HexA and M6PHexA by SDS-PAGE (A) and Western blotting (B). Purified HexA and M6PHexA were separated by 10% SDS-PAGE and analyzed by CBB staining (A) and immunostaining (B). The blotted membrane was overlaid with rabbit anti-human HexA serum, followed by alkaline phosphatase-conjugated anti-rabbit immunoglobulin G as the secondary antibody. CDP-Star detection reagent (GE Healthcare Bio-Sciences Corp.) was used to visualize the enzymes. M, molecular marker; lane 1, HexA; lane 2, M6PHexA.
Enzymatic activities towards artificial substrates and GM2 ganglioside.
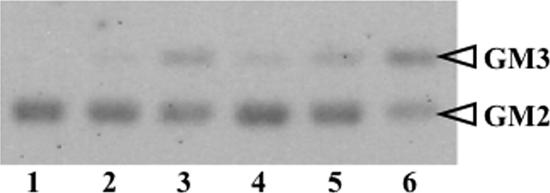
The specific activities of recombinant HexA for MUG, MUGS, and pNP-GlcNAc were 1.2 ± 0.2, 0.5 ± 0.01, and 1.5 ± 0.1 mmol/h/mg (mean ± standard error), respectively, and those of M6PHexA were 1.7 ± 0.3, 0.7 ± 0.1, and 2.6 ± 0.2 mmol/h/mg, respectively. The activity towards native substrate, GM2 ganglioside, was detected under in vitro conditions using sodium taurodeoxycholate as a detergent in place of GM2 activator protein (23). Recombinant HexA and desalted human placenta β-hexosaminidase with an activity of 300 nmol/h (toward MUGS) were incubated with GM2 ganglioside at 37°C for 1, 3, and 24 h. The TLC pattern for hydrolysis showed that the recombinant HexA hydrolyzed GM2 ganglioside and yielded GM3 ganglioside in the same manner as the human enzyme (Fig. 3). The complete degradation of GM2 ganglioside (8 μg) was observed within 24 h when HexA with a MUGS-hydrolyzing activity of 500 nmol/h was added to the reaction mixture (data not shown).
FIG. 3.
GM2 ganglioside hydrolysis by recombinant HexA. Applied samples are as follows: 1, GM2 standard (GM2 1.2 μg plus sodium taurodeoxycholate [TD], 30 μg); 2, GM2 plus TD plus human placenta β-hexosaminidase for a 3-h incubation; 3, GM2 plus TD plus human placenta β-hexosaminidase for a 24-h incubation; 4, GM2 plus TD plus HexA for a 1-h incubation; 5, GM2 plus TD plus HexA for a 3-h incubation; 6, GM2 plus TD plus HexA for a 24-h incubation. The enzymatic reaction product by recombinant HexA produced in O. minuta was identified as GM3 based on the Rf value on TLC, which was identical with that of the authentic human placenta-derived β-hexosaminidase enzyme.
Exposure and content of M6P on recombinant HexA.
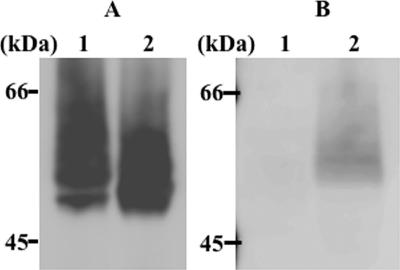
To detect M6P exposure on the N-glycan of M6PHexA and its recognition by the M6P receptor, immunostaining and receptor staining of HexA and M6PHexA were performed using anti-HexA antibody and Dom9His, respectively (Fig. 4). Of the 15 domains that compose the cation-independent M6P receptor, domain 9 demonstrates high affinity to M6P even when it is expressed alone as a truncated form (10, 11, 36). HexA was detected only by the anti-HexA antibody, while M6PHexA was detected by both the antibody and Dom9His (Fig. 4, lane 2). These results indicate that M6P residues on HexA N-glycan were properly uncovered by α-mannosidase treatment. However, some of the M6PHexA molecules, especially those of low molecular weight, were detected only by anti-HexA antibody and not by Dom9His (Fig. 4, lane 2). The M6PHexA molecules that were unrecognized by Dom9His probably do not contain acidic sugar (M6P) residues on their N-glycans; they probably lost most of the mannose residues on their N-glycans due to the α-mannosidase treatment.
FIG. 4.
Interaction between Dom9His and M6PHexA. Blotted recombinant enzymes were detected by anti-HexA antibody (A) and Dom9His (B), as described in Materials and Methods. Lane 1, HexA; lane 2, M6PHexA.
To determine the M6P content of HexA, N-glycans were enzymatically separated from HexA by using PNGase F and analyzed by HPLC (Table 2). About 6% of the total N-glycan was phosphorylated, while over 90% of the N-glycans were not phosphorylated. Regardless of phosphorylation, the length of mannose residues on HexA N-glycans varied from 8 (Man8GlcNAc2) to 15 (Man15GlcNAc2), with 10 mannose residues (Man10GlcNAc2) the most abundant.
TABLE 2.
Analysis of HexA N-glycana
| Sugar | % of total
|
|
|---|---|---|
| Neutral (nonphosphorylated) | Acidic (phosphorylated)b | |
| Man8GlcNAc2 | 14.2 | 1.1 |
| Man9GlcNAc2 | 15.9 | 1.4 |
| Man10GlcNAc2 | 22.9 | 1.5 |
| Man11GlcNAc2 | 21.5 | 1.2 |
| Man12GlcNAc2 | 9.8 | 0.5 |
| Man13GlcNAc2 | 3.5 | 0.2 |
| Man14GlcNAc2 | 1.9 | 0.2 |
| ≥Man15GlcNAc2 | 4.2 | ND |
| Total | 93.9 | 6.1 |
Man, mannose; ND, not detected. n = 3. The values were calculated from the peak areas under the assay conditions for HPLC, as described in Materials and Methods.
The acidic sugars include mannose-6-phosphate.
Uptake of M6PHexA by TS and SD fibroblasts and observation of intracellular GM2 ganglioside.
The incorporation of recombinant HexA into TS and SD fibroblasts was determined by restoration of MUGS-hydrolyzing activity in cell homogenates. The residual MUGS-hydrolyzing activities of HexA and M6PHexA incubated in the Ham's F-10 medium for 1 day at 37°C were 70% and 40%, respectively (data not shown). Ohsawa et al. (33) considered the low stability of HexA within the medium and replaced half of the medium every day with fresh medium that contained the appropriate concentration of enzyme throughout the uptake experiments. Because the residual activity of M6PHexA in the medium at 37°C was gradually reduced within 72 h of incubation (data not shown), we followed the procedures described by Ohsawa et al. (33). M6PHexA was incorporated into the TS and SD fibroblasts via M6P receptors, and its incorporation was inhibited by simultaneous addition of 5 mM M6P, while no incorporation was observed when HexA was added (Fig. 5A). These results suggest that HexA is incorporated into fibroblasts in an M6P receptor-dependent manner.
FIG. 5.
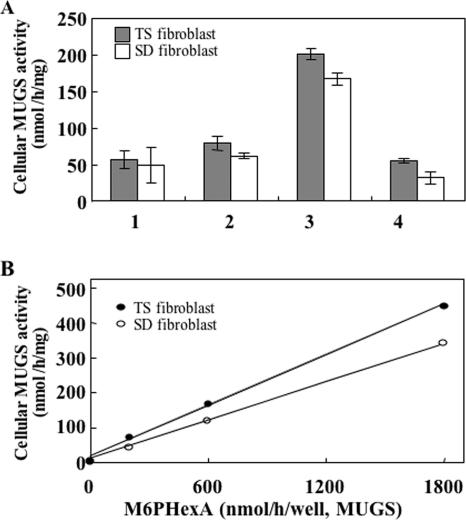
Analysis of M6PHexA uptake by TS and SD fibroblasts. (A) TS and SD fibroblasts were cultured in medium containing recombinant HexA enzymes (600 nmol/h/well, toward MUGS), and the MUGS-hydrolyzing activity of homogenates was measured to determine enzyme incorporation. 1, no enzyme addition; 2, HexA; 3, M6PHexA; 4, M6PHexA with 5 mM M6P. Each bar represents the mean of two independent experiments. The cellular MUGS-hydrolyzing activity of fibroblasts from a normal subject was 752 nmol/h/mg. Error bars represent standard errors of the means. (B) Dose dependency of enzyme uptake was determined by the addition of M6PHexA to the TS and SD fibroblasts at the MUGS-hydrolyzing activities of 200, 600, and 1,800 nmol/h/well. The MUGS-hydrolyzing activity of cell extracts was determined to detect enzyme incorporation.
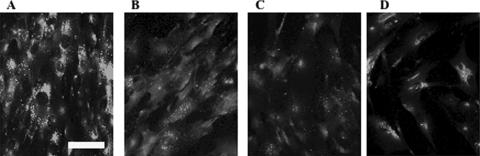
Dose-dependent incorporation of M6PHexA activity up to 1,800 nmol/h/well was observed for both TS and SD fibroblasts (Fig. 5B). The effect of incorporated M6PHexA on accumulated GM2 gangliosides in TS fibroblasts was observed by immunofluorescence analysis (Fig. 6). GM2 that accumulates in lysosomes and endosomes forms granular aggregates, which appear as spots during immunofluorescence analysis. Degradation of granular GM2 was observed dose dependently in M6PHexA-incorporated TS fibroblasts.
FIG. 6.
Analysis of intracellular GM2 ganglioside degradation in TS fibroblasts. After the TS fibroblasts were cultured in medium that contained various amounts of recombinant enzymes, they were fixed and intracellular GM2 gangliosides were detected by antibody to GM2 ganglioside. (A) TS fibroblasts; (B) TS fibroblasts plus M6PHexA (600 nmol/h/well); (C) TS fibroblasts plus M6PHexA (1,800 nmol/h/well); (D) normal fibroblasts. Bar, 10 μm.
DISCUSSION
We previously produced homodimeric α-GalA, a recombinant human lysosomal enzyme, in S. cerevisiae; this enzyme was suitable for enzyme replacement therapy of Fabry disease (8). In the present study, we produced recombinant human β-hexosaminidase A, a human lysosomal heterodimeric glycoprotein, in the methylotrophic yeast O. minuta for utilization in the treatment of TS and SD. The amount of recombinant β-hexosaminidase isozymes that were produced was 57 mg/liter of broth, and both HexA and M6PHexA were obtained with greater than 90% purity, as determined by HPLC analysis (data not shown). Several groups, including our group, have produced recombinant α-GalA in various hosts. The amounts of recombinant α-GalA produced were as follows: 0.019 mg/10-cm culture dish under the expression system of COS-7 cells (47), 4.8 mg/liter broth in Sf9 cells (6), 0.1 mg/liter broth in S. cerevisiae (8), and 4.5 mg/liter broth in P. pastoris (7). These results suggested that O. minuta has promising potential as a host for heterologous recombinant protein expression.
We found two major differences between recombinant HexA from O. minuta and native HexA from human lysosomes: the ratio of isozymes expressed and the processing patterns, especially for the β-subunit. The SDS-PAGE patterns of deglycosylated HexA suggest that the second processing site (R356QNK) of the β-subunit is not processed. Also, the deduced mass of the deglycosylated β-subunit band on the CBB staining (43 kDa) was smaller than the calculated mass (49.9 kDa). The C terminus of the β-subunit is possibly truncated in the O. minuta recombinant, probably due to protease digestion specific to the yeast cell, which is not observed in mammalian HexA. This truncation can explain the difference in the ratio of isozymes expressed. Since the amino acid residues in the C terminus of the β-subunit participate in subunit dimerization (22, 25, 26), their truncation reduces the area of dimer interface for recombinant HexB (ββ) and HexA (αβ) compared with that from mammalian cells. As a result, the truncation is likely to produce less-stable ββ and αβ dimers, which explains why HexB (ββ) is the minor isozyme and HexS (αα) is the major isozyme in O. minuta. Although the protease responsible for the C terminus truncation of the β-subunit has not been identified, protease-disrupted O. minuta strains, which we have already established, may prevent the C terminus truncation of the β-subunit, and the isozyme ratio may become closer to that of mammalian cells.
Reduced production of HexB, however, is advantageous for our yeast recombinant expression system, since recombinant HexA can be purified in one step by using a HisTrap column that binds to the His tag attached to the β-subunit. It appears that purified HexA (MUG/MUGS ratio, 2.6) does not contain HexB, because the MUG/MUGS ratios for recombinant HexA and HexB are about 3.5 to 4 and 200 to 300 in CHO cells, respectively (15, 44). In the case of recombinants from O. minuta, the MUG/MUGS ratio should exceed 4.0 if HexB is mixed in with purified HexA. Also, purified HexA was analyzed and confirmed not to contain other isozymes, as determined by HPLC and Western blotting of native PAGE gels with antibodies against HexA, the α-subunit, and the β-subunit.
Lysosomal targeting in ERT requires that HexA contain exposed M6P that can interact with cell surface receptors. As shown in Table 2, about 6% of the N-glycans were phosphorylated, and most of the recombinant HexA N-glycans did not contain mannosyl phosphate residues. Such nonphosphorylated N-glycan chains are shortened, theoretically, to Man1GlcNAc2 (Manβ1,4GlcNAcβ1,4GlcNAc) by α-mannosidase treatment, indicating that many M6PHexA molecules are glycosylated with only Man1GlcNAc2, with no M6P residues. Nevertheless, the successful incorporation of M6PHexA through M6P receptors was observed in both TS and SD fibroblasts in an M6P receptor-dependent manner (Fig. 5A). We anticipate that the dose dependency of M6PHexA activity continues over 1,800 nmol/h/well, since the ERT effect does not reach saturation at this concentration for either type of cell (Fig. 5B). The diminishing GM2 gangliosides in the TS fibroblasts (Fig. 6) suggest a proper interaction of recombinant M6PHexA with the intracellular GM2 activator protein for hydrolysis of accumulated GM2 gangliosides. These results indicate that recombinant HexA produced in O. minuta is suited for enzyme replacement therapy of GM2 gangliosidosis. Although a good effect for enzyme replacement is observed with a 6% M6P-containing HexA, it is necessary to distinguish the N-glycosylation sites, within the seven potential sites, that are attached with phosphorylated N-glycans. Generally, the stability of a glycoprotein decreases as the glycan content decreases. In fact, the stability of M6PHexA at 37°C in Ham's F-10 medium was about half the stability of HexA (data not shown). Therefore, a higher N-glycan content of M6PHexA, which means a higher M6P content, is required for the efficient incorporation of enzyme into the cell and especially for the stability of the enzyme.
Recombinant human α-GalA produced by S. cerevisiae KK4 (8) contained over 60% phosphorylated N-glycan due to the constitutive expression of the MNN4 gene (ScMNN4), which is a positive regulator of mannosylphosphate transferase in the host strain (30, 31). Increased M6P content in recombinant α-GalA was observed when it was expressed from an O. minuta strain that coexpressed ScMNN4 (unpublished data). This finding suggests that ScMNN4 functions in O. minuta as well as in S. cerevisiae. Recently, genes homologous to ScMNN4 were found in the O. minuta genome. The M6P content of recombinant HexA is expected to increase when ScMNN4 or the O. minuta homologous gene is coexpressed in the host strain. The enhanced M6P content prevents the generation of shorter N-glycans (Man1GlcNAc2) from α-mannosidase treatment and is expected to increase the stability of M6PHexA. Stable M6PHexA will lead to more efficient enzyme replacement and GM2 degradation; the increased efficiency may possibly reduce the number of treatments and the related discomfort for TS and SD patients when it is actually employed in clinical applications. Although the effect of ERT using O. minuta M6PHexA in a mouse model of SD should be further studied and increased M6P content should be established, we believe that the production of HexA in yeast is cost conscious and useful for ERT of GM2 gangliosidosis.
Acknowledgments
We thank Kousuke Kuroda and Toshihiro Komeda from Kirin Brewery Co., Ltd., for providing the O. minuta host strain and vectors, Richard L. Proia from the National Institutes of Health for providing the cDNA, and Yutaka Tatano from the University of Tokushima for helping with the purification of recombinant HexA and for useful discussions.
This work was supported by grants from CREST, JST.
Footnotes
Published ahead of print on 8 June 2007.
REFERENCES
- 1.Amalfitano, A., A. J. McVie-Wylie, H. Hu, T. L. Dawson, N. Rabens, P. Plotz, and Y. T. Chen. 1999. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-α-glucosidase. Proc. Natl. Acad. Sci. USA 96:8861-8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, U., D. Smith, M. Jeyakumar, T. D. Butters, M. C. Borja, R. A. Dwek, and F. M. Platt. 2004. Improved outcome of N-butyldeoxygalactonojirimycin-mediated substrate reduction therapy in a mouse model of Sandhoff disease. Neurobiol. Dis. 16:506-515. [DOI] [PubMed] [Google Scholar]
- 3.Asano, N., S. Ishii, H. Kizu, K. Ikeda, K. Yasuda, A. Kato, O. R. Maritn, and J. Q. Fan. 2000. In vivo inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur. J. Biochem. 267:4179-4186. [DOI] [PubMed] [Google Scholar]
- 4.Barton, N. W., R. O. Brady, J. M. Dambrosia, A. M. Di Bisceglie, S. H. Doppelt, S. C. Hill, H. J. Mankin, G. J. Murray, R. I. Parker, and C. E. Argoff. 1991. Replacement therapy for inherited enzyme deficiency-macrophage-medicated glucocerebrosidase for Gaucher's disease. N. Engl. J. Med. 324:1464-1470. [DOI] [PubMed] [Google Scholar]
- 5.Butters, T. D., R. A. Dwek, and F. M. Platt. 2000. Inhibition of glycosphingolipid biosynthesis: application to lysosomal storage disorders. Chem. Rev. 100:4683-4696. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., M. Jin, L. Goodrich, G. Smith, G. Coppola, and D. H. Calhoun. 2000. Purification and characterization of human α-galactosidase A expressed in insect cells using a baculovirus vector. Protein Expr. Purif. 20:228-236. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Y., M. Jin, T. Egborge, G. Coppola, J. Andre, and D. H. Calhoun. 2000. Expression and characterization of glycosylated and catalytically active recombinant human α-galactosidase A produced in Pichia pastoris. Protein Expr. Purif. 20:472-484. [DOI] [PubMed] [Google Scholar]
- 8.Chiba, Y., H. Sakuraba, M. Kotani, R. Kase, K. Kobayashi, M. Takeuchi, S. Ogasawara, Y. Maruyama, T. Nakajima, Y. Takaoka, and Y. Jigami. 2002. Production in yeast of α-galactosidase A, a lysosomal enzyme applicable to enzyme replacement therapy for Fabry disease. Glycobiology 12:821-828. [DOI] [PubMed] [Google Scholar]
- 9.Eng, C. M., N. Guffon, W. R. Wilcox, D. P. Germain, P. Lee, S. Waldek, L. Caplan, G. E. Linthrost, and R. J. Desnick. 2001. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N. Engl. J. Med. 345:55-57. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, M. K., D. J. Haskins, G. Sun, and N. M. Dahms. 2002. Identification of residues essential for carbohydrate recognition by the insulin-like growth factor II/mannose 6-phosphate receptor. J. Biol. Chem. 277:11255-11264. [DOI] [PubMed] [Google Scholar]
- 11.Hancock, M. K., R. D. Yammani, and N. M. Dahms. 2002. Localization of the carbohydrate recognition sites of the insulin-like growth factor II/mannose 6-phosphate receptor to domains 3 and 9 of the extracytoplasmic region. J. Biol. Chem. 277:47205-47212. [DOI] [PubMed] [Google Scholar]
- 12.Harmatz, P., C. B. Whitley, L. Waber, R. Pais, R. Steiner, B. Plecko, P. Kaplan, J. Simon, E. Butensky, and J. J. Hopwood. 2004. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J. Pediatr. 144:574-580. [DOI] [PubMed] [Google Scholar]
- 13.Hepbildikler, S. T., R. Sandhoff, M. Kolzer, R. L. Proia, and K. Sandhoff. 2002. Physiological substrates for human lysosomal β-hexosaminidase S. J. Biol. Chem. 277:2562-2572. [DOI] [PubMed] [Google Scholar]
- 14.Hoogerbrugge, P. M., B. J. H. M. Poorthuis, A. E. Romme, J. J. P. van de Kamp, G. Wagemaker, and D. W. van Bekkum. 1988. Effect of bone marrow transplantation on enzyme levels and clinical course in the neurologically affected twitcher mouse. J. Clin. Investig. 81:1790-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou, Y., R. Tse, and D. J. Mahuran. 1996. Direct determination of the substrate specificity of the α-active site in heterodimeric β-hexosaminidase A. Biochemistry 35:3963-3969. [DOI] [PubMed] [Google Scholar]
- 16.Ichisaka, S., K. Ohno, I. Yuasa, E. Nanba, H. Sakuraba, and Y. Suzuki. 1998. Increased expression of β-hexosaminidase α chain in cultured skin fibroblasts from patients with carbohydrate-deficient glycoprotein syndrome type I. Brain Dev. 20:302-306. [DOI] [PubMed] [Google Scholar]
- 17.Jung, S. C., I. P. Han, A. Limaye, R. Xu, M. P. Gelderman, P. Zerfas, K. Tirumalai, G. J. Murray, M. J. During, R. O. Brady, and P. Qasba. 2001. Adeno-associated viral vector-mediated gene transfer results in long-term enzymatic and functional correction in multiple organs of Fabry mice. Proc. Natl. Acad. Sci. USA 98:2676-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinge, L., V. Straub, U. Neudorf, and T. Voit. 2005. Enzyme replacement therapy in classical infantile Pompe disease: results of a ten-month follow-up study. Neuropediatrics 36:6-11. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda, K., K. Kobayashi, H. Tsumura, T. Komeda, Y. Chiba, and Y. Jigami. 2006. Production of Man5GlcNAc2-type sugar chain by the methylotrophic yeast Ogataea minuta. FEMS Yeast Res. 6:1052-1062. [DOI] [PubMed] [Google Scholar]
- 20.Kyrkanides, S., J. H. Miller, M. Brouxhon, J. A. Olschowka, and H. J. Federoff. 2005. β-Hexosaminidase lentiviral vectors: transfer into the CNS via systemic administration. Mol. Brain Res. 133:286-298. [DOI] [PubMed] [Google Scholar]
- 21.Lau, M. M. H., and E. F. Neufeld. 1989. A frameshift mutation in a patient with Tay-Sachs disease causes premature termination and defective intracellular transport of the α-subunit of β-hexosaminidase. J. Biol. Chem. 264:21376-21380. [PubMed] [Google Scholar]
- 22.Lemieux, M. J., B. L. Mark, M. M. Cherney, S. G. Withers, D. J. Mahuran, and M. N. G. James. 2006. Crystallographic structure of human β-hexosaminidase A: interpretation of Tay-Sachs mutations and loss of GM2 ganglioside hydrolysis. J. Mol. Biol. 359:913-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, S. C., S. Serizawa, and Y. T. Li. 1984. Effect of modification of sialic acid on enzymatic hydrolysis of ganglioside GM1 and GM2. J. Biol. Chem. 259:5409-5410. [PubMed] [Google Scholar]
- 24.Little, L. E., M. M. H. Lau, D. V. K. Quon, A. V. Fowler, and E. F. Neufeld. 1988. Proteolytic processing of the α-chain of the lysosomal enzyme, β-hexosaminidase, in normal human fibroblasts. J. Biol. Chem. 263:4288-4292. [PubMed] [Google Scholar]
- 25.Maier, T., N. Strater, C. G. Schuette, R. Klingenstein, K. Sandhoff, and W. Saenger. 2003. The X-ray crystal structure of human β-hexosaminidase B provides new insights into Sandhoff disease. J. Mol. Biol. 328:669-681. [DOI] [PubMed] [Google Scholar]
- 26.Mark, B. L., D. J. Mahuran, M. M. Cherney, D. Zhao, S. Knapp, and M. N. G. James. 2003. Crystal structure of human β-hexosaminidase B: understanding the molecular basis of Sandhoff and Tay-Sachs disease. J. Mol. Biol. 327:1093-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meier, E. M., G. Schwarzmann, W. Furst, and K. Sandhoff. 1991. The human GM2 activator protein. J. Biol. Chem. 266:1879-1887. [PubMed] [Google Scholar]
- 28.Muenzer, J., J. C. Lamsa, A. Garcia, J. Dacosta, J. Garcia, and D. A. Treco. 2002. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr. Suppl. 91:98-99. [DOI] [PubMed] [Google Scholar]
- 29.Norflus, F., C. J. Tifft, M. P. McDonald, G. Goldstein, J. N. Crawley, A. Hoffmann, K. Sandhoff, K. Suzuki, and R. L. Proia. 1998. Bone marrow transplantation prolongs life span and ameliorates neurologic manifestations in Sandhoff disease mice. J. Clin. Investig. 101:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odani, T., Y. Shimma, A. Tanaka, and Y. Jigami. 1996. Cloning and analysis of the MNN4 gene required for phosphorylation of N-linked oligosaccharides in Saccharomyces cerevisiae. Glycobiology 6:805-810. [DOI] [PubMed] [Google Scholar]
- 31.Odani, T., Y. Shimma, X. H. Wang, and Y. Jigami. 1997. Mannosylphosphate transfer to cell wall mannan is regulated by the transcriptional level of the MNN4 gene in Saccharomyces cerevisiae. FEBS Lett. 420:186-190. [DOI] [PubMed] [Google Scholar]
- 32.O'Dowd, B. F., M. H. Klavins, H. F. Willard, R. Grabel, J. A. Lowden, and D. J. Mahuran. 1986. Molecular heterogeneity in the infantile and juvenile forms of Sandhoff disease (O-variant GM2 gangliosidosis). J. Biol. Chem. 261:12680-12685. [PubMed] [Google Scholar]
- 33.Ohsawa, M., M. Kotani, Y. Tajima, D. Tsuji, Y. Ishibashi, A. Kuroki, K. Itoh, K. Watabe, K. Sango, S. Yamanaka, and H. Sakuraba. 2005. Establishment of immortalized Schwann cells from Sandhoff mice and corrective effect of recombinant human β-hexosaminidase A on the accumulated GM2 ganglioside. J. Hum. Genet. 50:460-467. [DOI] [PubMed] [Google Scholar]
- 34.Potier, M., L. Mameil, M. Belisle, L. Dallaine, and S. B. Melancon. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-d-N-acetylneuraminate) substrate. Anal. Biochem. 94:287-296. [DOI] [PubMed] [Google Scholar]
- 35.Quon, D. V. K., R. L. Proia, A. V. Fowler, J. Bleibaum, and E. F. Neufeld. 1989. Proteolytic processing of the β-subunit of the lysosomal enzyme, β-hexosaminidase, in normal human fibroblasts. J. Biol. Chem. 264:3380-3384. [PubMed] [Google Scholar]
- 36.Reddy, S. T., W. Chai, R. A. Childs, J. D. Page, T. Feizi, and N. M. Dahms. 2004. Identification of a low affinity mannose 6-phosphate-binding site in domain 5 of the cation-independent mannose 6-phosphate receptor. J. Biol. Chem. 279:38658-38667. [DOI] [PubMed] [Google Scholar]
- 37.Sakuraba, H., Y. Chiba, M. Kotani, I. Kawashima, M. Ohsawa, Y. Tajima, Y. Takaoka, Y. Jigami, H. Takahashi, Y. Hirai, T. Shimada, Y. Hashimoto, K. Ishii, T. Kobayashi, K. Watabe, T. Fukushige, and T. Kanzaki. 2006. Corrective effect on Fabry mice of yeast recombinant human α-galactosidase with N-linked sugar chains suitable for lysosomal delivery. J. Hum. Genet. 51:341-352. [DOI] [PubMed] [Google Scholar]
- 38.Schiffmann, R., G. J. Murray, D. Treco, P. Daniel, M. Sellos-Moura, M. Myers, J. M. Quirk, G. C. Zirzow, M. Borowski, K. Loveday, T. Anderson, F. Gillespie, K. L. Oliver, N. O. Jeffries, E. Doo, T. J. Liang, C. Kreps, K. Gunter, K. Frei, K. Crutchfield, R. F. Selden, and R. O. Brady. 2000. Infusion of α-galactosidase A reduces tissue globotriaosylceremide storage in patients with Fabry disease. Proc. Natl. Acad. Sci. USA 97:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein, C. S., A. Ghodsi, T. Derksen, and B. L. Davidson. 1999. Systemic and central nervous system correction of lysosomal storage in mucopolysaccharidosis type VII mice. J. Virol. 73:3424-3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takashiba, M., Y. Chiba, and Y. Jigami. 2006. Identification of phosphorylation sites in N-linked glycans by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 78:5208-5213. [DOI] [PubMed] [Google Scholar]
- 41.Tarentino, A. L., and F. Maley. 1972. β-N-Acetylglucosaminidase from hen oviduct. Methods Enzymol. 28:772-776. [DOI] [PubMed] [Google Scholar]
- 42.Tropak, M. B., S. P. Reid, M. Guiral, S. G. Withers, and D. Mahuran. 2004. Pharmacological enhancement of β-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff patients. J. Biol. Chem. 279:13478-13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji, A., K. Omura, and Y. Suzuki. 1988. Intracellular transport of acid α-glucosidase in human fibroblasts: evidence for involvement of phosphomannosyl receptor-independent system. J. Biochem. 104:276-278. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji, D., A. Kuroki, Y. Ishibashi, T. Itakura, and K. Itoh. 2005. Metabolic correction in microglia derived from Sandhoff disease model mice. J. Neurochem. 94:1631-1638. [DOI] [PubMed] [Google Scholar]
- 45.Van den Hout, J. M. P., J. H. J. Kamphoven, L. P. F. Winkel, W. F. M. Arts, J. B. C. de Klerk, M. C. B. Loonen, A. G. Vulto, A. Cromme-Dijkhuis, N. Weisglas-Kuperus, W. Hop, H. van Hirtum, O. P. van Diggelen, M. Boer, M. A. Kroos, P. A. van Doorn, E. van der Voort, B. Sibbles, E. J. J. M. van Corven, J. P. J. Brakenhoff, J. van Hove, J. A. M. Smeitink, G. de Jong, A. J. J. Reuser, and A. T. van der Ploeg. 2004. Long-term intravenous treatment of Pompe disease with recombinant human α-glucosidase from milk. Pediatrics 113:e448-e457. [DOI] [PubMed] [Google Scholar]
- 46.Wraith, J. E., L. A. Clarke, M. Beck, E. H. Kolodny, G. M. Pastores, J. Muenzer, D. M. Rapoport, K. I. Berger, S. J. Sweidler, E. D. Kakkis, T. Braakman, E. Chadbourne, K. Walton-Bowen, and G. F. Cox. 2004. Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-l-iduronidase (laronidase). J. Pediatr. 144:581-588. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda, K., H. H. Chang, H. L. Wu, S. Ishii, and J. Q. Fan. 2004. Efficient and rapid purification of recombinant human α-galacotosidase A by affinity column chromatography. Protein Expr. Purif. 37:499-506. [DOI] [PubMed] [Google Scholar]
- 48.Yuziuk, J. A., C. Bertoni, T. Beccari, A. Orlacchio, Y. Y. Wu, S. C. Li, and Y. T. Li. 1998. Specificity of mouse GM2 activator protein and β-N-acetylhexosaminidases A and B. J. Biol. Chem. 273:66-72. [DOI] [PubMed] [Google Scholar]