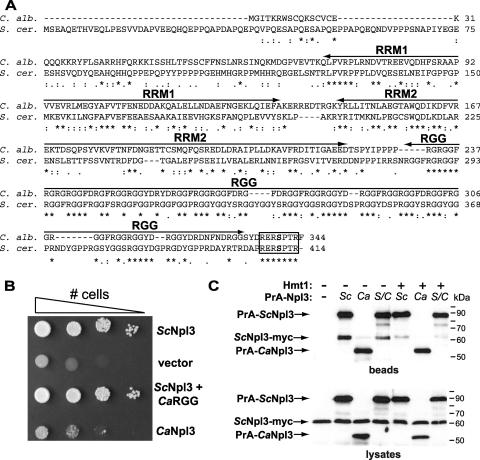
FIG. 4.
The RGG domain of C. albicans Npl3, but not full-length protein, can function in S. cerevisiae. (A) Clustal W alignment (9) of S. cerevisiae RNA-binding protein Npl3 (S. cer.) and the C. albicans Npl3 ortholog (C. alb.). RNA recognition motifs (RRMs) and arginine-glycine-rich (RGG) domains are shown. Sky1-mediated phosphorylation of serine 411 (bold) in the conserved C-terminal heptapeptide facilitates Npl3 nuclear import in S. cerevisiae (20, 50). SR dipeptides found in S. cerevisiae, but not C. albicans, Npl3 are underlined. Asterisks denote identical residues in all sequences, colons denote conserved substitutions, and periods denote semiconserved substitutions. (B) An S. cerevisiae strain lacking NPL3 (PSY814) was transformed with plasmids that express PrA alone (vector; pNOPPATA) or PrA fused to S. cerevisiae Npl3 (ScNpl3; pPS2389), C. albicans Npl3 (CaNpl3; pAM361), or a chimeric protein composed of the first three domains of ScNpl3 with the RGG domain of CaNpl3 (ScNpl3-CaRGG; pAM362). Cells were grown overnight to mid-log phase in medium lacking leucine and washed, and 10-fold dilutions (106 to 103 cells) were plated on 5-FOA medium and grown at 30°C for 2 days. (C) hmt1Δ (YAM533) and HMT1 (YAM535) S. cerevisiae strains expressing myc-tagged ScNpl3 were transformed with the PrA-Npl3 plasmids shown in panel B. Proteins from mid-log-phase cells were precipitated with IgG-Sepharose. Proteins isolated from 1.7 mg lysate (beads) and total lysates (20 μg) were analyzed by immunoblotting with a polyclonal anti-myc antiserum, which also binds to PrA.