FIG. 2.
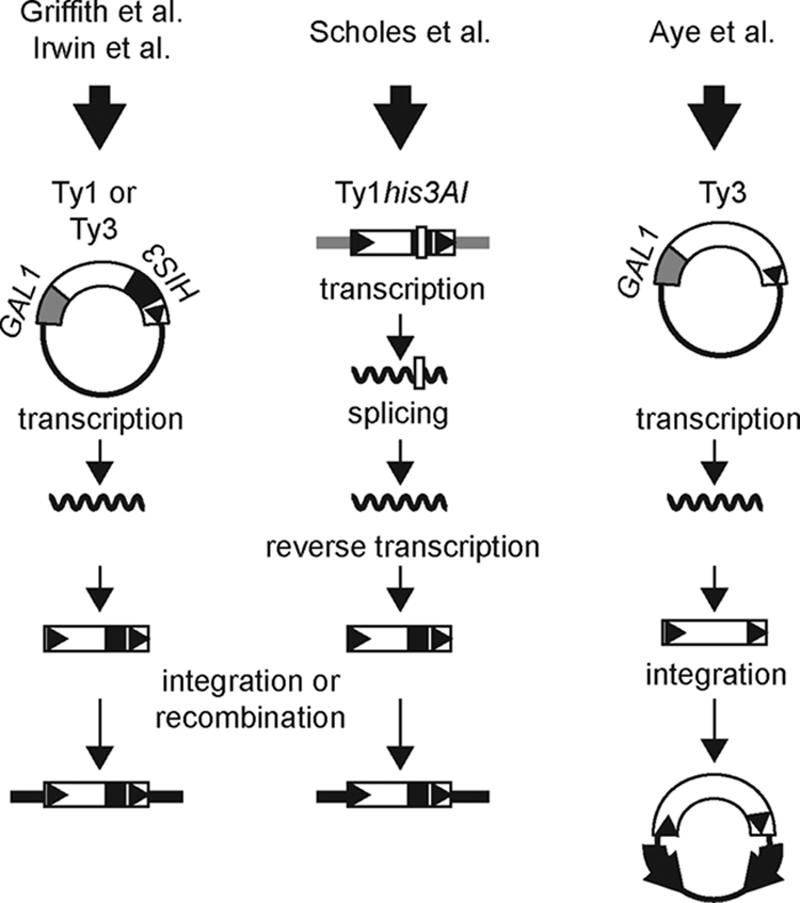
Comparison of Ty mobility assays used in the four high-throughput screens for Ty regulators. The three columns are schematic representations of the different experimental approaches used to measure Ty mobility in the primary screen of each of four studies. The column on the left depicts a plasmid copy of a Ty element expressed from the GAL1 promoter and marked with a HIS3 reporter gene. The TyHIS3 element is transcribed and then reverse transcribed to produce a TyHIS3 cDNA that is inserted into the genome through Ty IN-mediated integration or host-mediated recombination with genomic Ty elements or LTRs. New TyHIS3 mobility events are detected by selecting for plasmid loss and the presence of a genomic copy of HIS3. The middle column depicts a genomic copy of a Ty1his3AI element in which a HIS3 marker gene is disrupted by an artificial intron (AI). Following transcription from the Ty1 promoter, the AI is spliced out of the Ty1his3AI transcript. After reverse transcription and insertion of the resulting cDNA molecule into the genome through integration or recombination with genomic Ty1 sequences, a functional HIS3 gene is restored, allowing new Ty1HIS3 mobility events to be detected. The column on the right depicts a plasmid copy of Ty3 expressed from the GAL1 promoter. The Ty3 element is transcribed, and the Ty3 transcript is reverse transcribed to produce a cDNA. Integration of the cDNA into a target locus, which consists of two closely spaced, divergently transcribed tRNA genes (block arrows) on a plasmid, is detected by relief of transcriptional repression of the tRNA genes, resulting in expression of reporter genes that require the corresponding tRNA suppressors.
