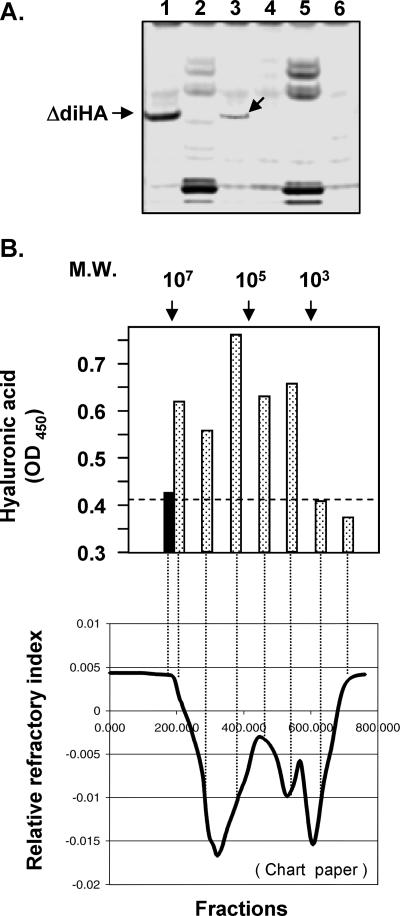
FIG. 7.
Characterization of C. neoformans capsule polysaccharide fractions. (A) FACE analysis. 2-Aminoacridone-derivatized products from C. neoformans capsular polysaccharide samples were resolved by electrophoresis. Lane 1, 5 μg hyaluronic acid as a standard marker; lane 2, B-4500FO2 crude capsular polysaccharide preparation; lane 3, B-4500FO2 Dowex 1 (2X) elution fraction (0.5 to 0.65 M NaCl); lane 4, enzyme/buffer control; lane 5, C559 (Δcps1) crude capsular polysaccharide preparation; lane 6, C559 Dowex 1 (2X) fraction (0.5 to 0.65 M NaCl fraction). An arrow in lane 3 indicates the band comigrating with a digested hyaluronic acid disaccharide unit (ΔdiHA). (B) Gel permeation using a Bio-Rad SEC-1000 size fractionation column to fractionate partially purified hyaluronic acid, followed by a hyaluronic acid ELISA. (Upper panel) Molecular weight (M.W.) markers are indicated at the top. Solid bar, blank control; stippled bars, aliquots (100 μl) from different fractions subjected to hyaluronic acid ELISA analysis. (Lower panel) Fractions corresponding to points on the curve on the refractor chart paper are shown (lower panel).