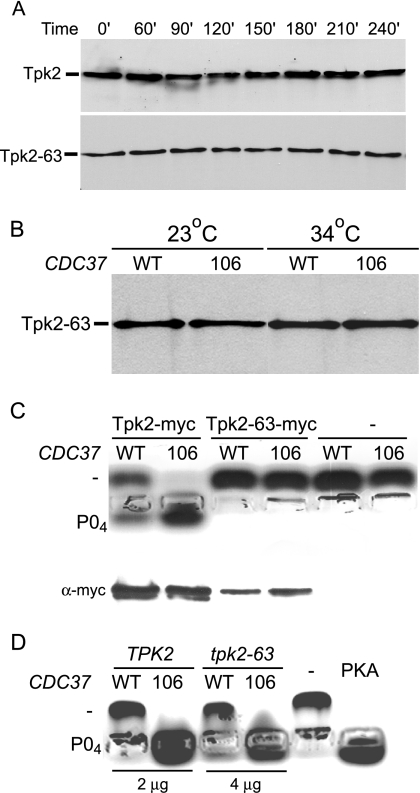
FIG. 4.
The CDC37 dominant mutations alter Tpk2 activity. (A) Stability of wild-type and temperature-sensitive Tpk2 proteins. Strains containing Myc-tagged wild-type Tpk2 and mutant Tpk2-63 fusion proteins were grown at 23°C and shifted to 34°C medium containing 100 mg/ml cycloheximide. Protein extracts were separated by polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and probed with anti-Myc antibody. Tpk2 (MRY142) and Tpk2-63 (MRY143) strains were used. (B) Dominant CDC37 mutations do not alter Tpk2 levels. Levels of the Myc-tagged temperature-sensitive Tpk2-63 fusion protein were determined after shifting derivatives of strain MRY142 from 23°C to 34°C for 3 h. Derivatives contained plasmids with either wild-type (WT) (CDC37+) (pYZ1) or mutant 106 (CDC37-106) (pGS224) alleles of CDC37. Extracts were separated by polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and probed with anti-Myc antibody to measure Tpk2 levels. (C) Dominant CDC37 mutations alter the activity of purified Tpk2. Tpk2 fusion proteins were immunopurified and used in an in vitro peptide kinase assay. After 30 min, peptide from each assay was separated by agarose gel electrophoresis to determine if the peptide was unphosphorylated (−) or phosphorylated (PO4). Aliquots of the immunoprecipitate were also separated by polyacrylamide gel electrophoresis, transferred onto nitrocellulose, and probed with anti-Myc antibody (α-myc) to determine if similar levels of protein kinase were present in each reaction. Strains were derivatives of Tpk2-Myc (MRY143) or Tpk2-63-Myc (MRY42) strains containing plasmids with WT (CDC37+ pYZ1) or 106 (CDC37 pGS224) alleles of CDC37. (D) Dominant CDC37 mutations alter the activity of wild-type and mutant PKA. Cell extracts from the indicated strains were used in the in vitro peptide kinase assay. TPK2 CDC37+ [SGY559(pRS316)], TPK2 CDC37-106 [SGY559(pYZ2)], tpk-63(Ts) CDC37+ [SGY446(pRS316)], and tpk-63(Ts) CDC37-106 [SGY446(pYZ2)] strains were used.