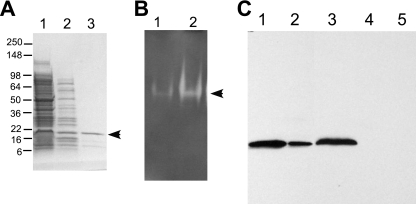
FIG. 2.
Cu,Zn SOD purification and activity. (A) Purification of Cu,Zn SOD from A. actinomycetemcomitans cell extract. Lanes: 1, strain JP2 cell extract after sonication (4 μg); 2, JP2 soluble extract (4 μg) after incubation at 60°C for 1 h; 3, Talon column eluate (2.5 μg). The gel was stained with Coomassie blue. (B) NBT SOD activity assay. Protein samples were resolved by native PAGE and stained with NBT. Lanes: 1, strain JP2 cell extract (2 μg); 2, purified Cu,Zn SOD (2.5 μg). (C) Isolation of and Western blot analysis with anti-Cu,Zn SOD antibody. Lanes: 1, purified Cu,Zn SOD (5 μg); 2, purified Cu,Zn SOD (0.5 μg); 3, JP2 cell extract (4 μg); 4, E. coli cell extract (5 μg); 5, bovine Cu,Zn SOD (1 μg). Molecular mass standards are in kDa. The arrows indicate the position of Cu,Zn, SOD.