Abstract
Poliovirus (PV) is easily transferred to humans orally; however, no rodent model for oral infections has been developed because of the alimentary tract's low sensitivity to the virus. Here we showed that PV is inactivated by the low pH of the gastric contents in mice. The addition of 3% NaHCO3 to the viral inoculum increased the titer of virus reaching the small intestine through the stomach after intragastric inoculation of PV. Transgenic mice (Tg) carrying the human PV receptor (hPVR/CD155) gene and lacking the alpha/beta interferon receptor (IFNAR) gene (hPVR-Tg/IfnarKO) were sensitive to the oral administration of PV with 3% NaHCO3, whereas hPVR-Tg expressing IFNAR were much less sensitive. The virus was detected in the epithelia of the small intestine and proliferated in the alimentary tract of hPVR-Tg/IfnarKO. By the ninth day after the administration of a virulent PV, the mice had died. These results suggest that IFNAR plays an important role in determining permissivity in the alimentary tract as well as the generation of virus-specific immune responses to PV via the oral route. Thus, hPVR-Tg/IfnarKO are considered to be the first oral infection model for PV, although levels of anti-PV antibodies were not elevated dramatically in serum and intestinal secretions of surviving mice when hPVR-Tg/IfnarKO were administered an attenuated PV.
Poliomyelitis is an acute disease of the central nervous system (CNS) caused by poliovirus (PV), a human enterovirus that belongs to the family Picornaviridae. In humans, an infection is initiated by oral ingestion of the virus, followed by multiplication in the alimentary mucosa (2, 38), from which the virus spreads through the bloodstream. Viremia is considered essential for leading to paralytic poliomyelitis in humans. By use of a PV-sensitive mouse model, previous studies (9, 44) demonstrated that after intravenous inoculation, circulating PV crosses the blood-brain barrier at a high rate, and a neural dissemination pathway from the skeletal muscle without injury is not the primary route by which the circulating virus disseminates to the CNS. Along with the blood-brain barrier pathway of dissemination, a neural pathway has been reported for humans (30), primates (11), and PV-sensitive transgenic mice (Tg) carrying the human PV receptor (hPVR/CD155) gene (31, 34); this pathway appears to be important in causing provocation poliomyelitis (9).
It has been proved that Tg carrying the hPVR gene (hPVR-Tg) are susceptible to all three PV serotypes, 1, 2, and 3 (22, 35), although mice without the hPVR gene are generally not susceptible to PV. This observation indicates that hPVR is the most important determinant of the host range of PV. After inoculation with PV by the intracerebral, intraspinal, intravenous, or intramuscular route (10, 20-22, 33-35), hPVR-Tg develop a flaccid paralysis in their limbs, which is clinically similar to human poliomyelitis. However, in contrast to its behavior in humans, PV does not replicate in the alimentary tracts of hPVR-Tg after oral administration, even in animals expressing high levels of hPVR in the intestinal epithelial cells (45). This result suggests that the expression of hPVR in the intestine is not solely responsible for the infection. It is also known that nonhuman primates are highly susceptible to PV by all routes except the oral route, yet the degree of oral susceptibility depends on the species (12). Thus, although oral infection is the most important route in humans, no adequate animal model has been established so far.
After an oral infection with PV, the virus must overcome at least three barriers before it can start to replicate efficiently in the first target cells in the small intestine: (i) the gastric acid solution, by which PV may be inactivated; (ii) inappropriate distribution of hPVR, by which PV may not be ushered to the correct target cells; and (iii) innate immunity, including interferon (IFN) signaling, by which the replication of PV may be hampered in the target cells (7). To know why orally administered PV hardly causes any paralysis in animals other than humans, we have to verify each step (see Fig. 7). In this report, barrier ii is defined as cell susceptibility and barrier iii is defined as cell permissivity.
FIG. 7.
Presumed PV dissemination routes and characteristics. The presumed barriers to orally ingested PV are shown. First, the ingested virus enters the stomach and suffers a low-pH environment (the first barrier). Second, the virus has to find the appropriate receptor in order to enter the intestinal cells (the second barrier, cell susceptibility). Third, the virus can start replicating in the cells depending on the lack of an IFN system (the third barrier, cell permissivity). Finally, the virus probably invades the bloodstream.
To control poliomyelitis, attenuated PV strains of all three serotypes have been developed and used effectively as oral polio vaccines (37, 39). The attenuated Sabin strains can replicate well only in the alimentary tracts of humans without showing neuropathogenicity, enough to elicit neutralizing antibodies against PV after oral administration.
Picornaviruses are sensitive to IFNs (3, 5, 24, 28, 46). IFNs play an essential role in the innate immune antiviral response. Recently, Ida-Hosonuma et al. (13) found that deletion of the IFN-α/β receptor (IFNAR) gene in hPVR-Tg (hPVR-Tg/IfnarKO) resulted in the disruption of IFN-α/β signaling (27), which is an important determinant of the tissue tropism and pathogenicity of PV. Similarly, it has been reported that IFN-α/β plays an important role in the pathogenicity and tissue tropism of some viruses, including coxsackievirus and Theiler's virus in the Picornaviridae (6, 8, 26, 36, 42). These results suggest that not only hPVR (cell susceptibility) but also IFN-α/β (cell permissivity) contributes to the pathogenicity and tissue tropism of PV.
In this paper, we have clarified the instability of the virus in the gastric environment, where the low pH of the gastric contents inactivates PV. Furthermore, using hPVR-Tg with or without IFNAR expression, we have shown that IFN-α/β plays a key role in preventing PV from replicating in the intestines of mice.
MATERIALS AND METHODS
Viruses and cells.
The virulent Mahoney strain [PV1(M)OM] and the avirulent Sabin 1 strain [PV1(Sab)IC-0] of type 1 PV derived from infectious cDNA clones pOM1 (41) and pVS(1)IC-0(T) (19), respectively, were employed in this study. As other virulent strains, Lansing (type 2) and Leon (type 3) were used.
African green monkey kidney (AGMK) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% newborn calf serum and were used for the preparation of viruses, transfection with infectious cDNA clones, and plaque assays.
Tg.
The Tg strains used in this paper have been described previously (13). In brief, mice of a transgenic strain, ICR-PVRTg21 (21, 22), were backcrossed with C57BL/6 mice, and homozygotes with the C57BL/6 background (C57BL/6-PVRTg21) were produced. In this report, strain C57BL/6-PVRTg21 is referred to as PVRTg21. A129 mice, deficient in the Ifnar gene (27), were backcrossed with C57BL/6 mice and then further crossed with PVRTg21 or MPVRTg25-61 (MPVRTg25) (43). MPVRTg25 express hPVR under the control of the mouse PVR homolog (MPH) (25) regulatory gene. PVR+/+ Ifnar−/− mice were obtained by intercrossing these PVRTg21/IfnarKO (13) or MPVRTg25/IfnarKO. All mice used were free of specific pathogens and were 7 to 10 weeks old. Mice were treated according to the guidelines for the Care and Use of Laboratory Animals of The University of Tokyo.
Assay of PV inactivation.
PV1(M)OM (5 μl) was incubated at 37°C or 0°C for the periods indicated with 45 μl of each solution, and then the titer of virus was determined by a plaque assay. The pH 1 solution was 0.1 N HCl, the pH 2 solution was 0.01 N HCl, the pH 3 solution was 0.001 N HCl, and the pH 7 solution was saline. The pH of each solution was measured using pH test paper at the start and end points of the incubation.
Administration of viruses.
For sampling of the gastric contents, mice were fasted overnight and then anesthetized with an intraperitoneal injection of 300 to 400 μl of ketamine (10 mg/ml) and xylazine (0.2 mg/ml) in saline. The stomach was exposed, and the pylorus was ligated with silk thread. The mice were inoculated with 200 μl of saline by using a gastric tube, and the gastric contents were collected. The gastric contents were centrifuged at 15,000 rpm for 10 min, and the supernatant was filtered. The filtrate was used for the experiments.
The viruses were administered orally using quantitative water bottles (Drinko-Measurer DM-G1; O'Hara & Co., Ltd., Japan). First, mice were fasted overnight, and then 2 ml of a viral solution containing 3% NaHCO3 per mouse was administered within 24 h using the water bottles. The time point for starting the administration was taken as time zero. Twenty-four hours after the administration was started, the quantitative water bottles were replaced with conventional water bottles.
Recovery of viruses from tissues.
For determination of the titer of virus in the tissues, the mice inoculated with the viruses were anesthetized and whole blood was recovered from the right ventricle. Immediately, the mice were perfused with saline through the left ventricle, and the tissues were excised. The tissues were homogenized in DMEM without serum to prepare a 10% emulsion. The homogenates were centrifuged to remove any debris, and the supernatant containing the virus was subjected to a plaque assay.
Labeling of PV.
PV was purified by a protocol described previously (16). HeLa S3 cells were infected with PV1(M)OM at a multiplicity of infection of 10. The cells were harvested at 7 h postinfection, and the virus was purified from cytoplasmic extracts of the infected cells by using DEAE-Sepharose CL-6B (GE Healthcare Bio-Sciences KK) followed by centrifugation on a sucrose density gradient and CsCl equilibrium centrifugation. Purified virus was desalted by gel filtration on a PD-10 column (GE Healthcare Bio-Sciences KK) equilibrated with phosphate-buffered saline [PBS(−)] (per liter, 8.00 g NaCl, 1.15 g Na2HPO4, 0.20 g KCl, 0.10 g MgCl2·6H2O, 0.20 g KH2PO4 [pH 7.4]). The concentration of poliovirions was determined by measuring the absorbance at 260 nm, where 1.0 optical density unit is regarded as equivalent to 9.4 × 1012 virions. The labeling of the virus is based on a protocol kindly provided by Lucas Pelkmans (32). PV (0.4 mg at 0.4 mg/ml) was labeled with 0.39 μl of Alexa Fluor 555-succinimidyl ester (10 mg/ml in dimethyl sulfoxide) according to the manufacturer's instructions (Invitrogen). These fluorophores react exclusively with free amines, resulting in a stable carboxamide bond. Labeled virus was repurified with NAP5 columns (GE Healthcare Bio-Sciences KK), dialyzed against PBS(−), and stored at −80°C. The labeling ratio was 14 mol of dye per mol of virus, and the specific infectivity of labeled virus was not reduced.
Detection of fluorescently labeled virus.
After the MPVRTg25/IfnarKO were anesthetized as described above, the intestines were exposed. The small intestine was ligated with silk thread at two points. Labeled virus was injected into the small intestine between the knots of the thread. One hour later, the injected portion of the small intestine was excised and washed with saline. The small intestine was directly observed under a confocal laser scanning microscope (LSM510; Carl Zeiss MicroImaging GmbH). For preparation of fixed tissue sections, the small intestine was immediately frozen in OCT compound (Sakura Fine Technical Co., Ltd.). Tissue sections were prepared by using a Jung CM3000 cryostat (Leica Instruments GmbH), mounted on 3-aminopropyltriethoxysilane-coated slides (Matsunami Glass Industries, Ltd.), and dried. All the staining procedures were performed at room temperature. First, the specimens were fixed in PBS(−) containing 2% paraformaldehyde for 1 min and washed four times in PBS(−). After treatment with 1.5% normal goat serum in PBS(−) for 20 min, 1 μg/ml of fluorescein isothiocyanate-labeled Ulex europaeus agglutinin-1 (UEA-1) was applied, and the specimens were incubated for 15 min and then washed with PBS(−). Nucleic acids were stained with 50 nM SYTO59 (Invitrogen). The sections were mounted with 80% (vol/vol) glycerol in PBS(−) and analyzed with a confocal laser scanning microscope.
Neutralizing assay.
PVRTg21 and PVRTg21/IfnarKO were orally administered 3 × 108 PFU of Sabin 1 along with 3% NaHCO3 within 37 h by using quantitative water bottles. As a positive control, PVRTg21/IfnarKO were intravenously injected with 1 × 105 PFU of Sabin 1. Twenty-one days after the administration, whole blood was collected from each mouse and serum was prepared after centrifugation. After incubation of the serum at 45°C for 1 h, 100 PFU of Sabin 1 in 100 μl was mixed with 100 μl of serially diluted serum and incubated for 1 h at 37°C. The virus-serum mixture was subjected to a plaque assay. Neutralizing activity is expressed as the maximum denominator for the dilution that can neutralize 100 PFU of Sabin 1.
RESULTS
Mouse gastric contents can inactivate PV.
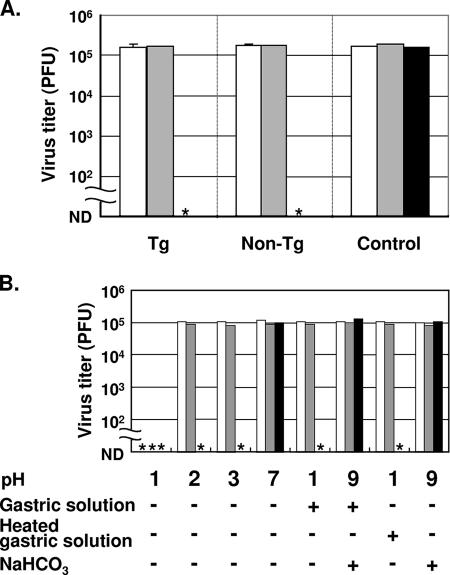
hPVR-Tg are much less susceptible to orally administered PV than humans. To explain this, we examined the stability of the virus during its passage through the mouse stomach after oral administration of PV. First, we examined whether a mouse gastric solution can inactivate PV. Under anesthesia, the pylorus was ligated and 200 μl of saline was inoculated using a gastric tube. Right after the inoculation, the gastric contents were collected, and the supernatant obtained by centrifugation was used as a gastric solution. As shown in Fig. 1A, 2 × 105 PFU of PV1(M)OM was incubated at 37°C or 0°C for the times indicated with or without the gastric solution from Tg or non-Tg. When PV was incubated at 37°C with the gastric solution from Tg or non-Tg, the titer of the virus was apparently reduced, whereas PV incubated with saline at 37°C for 4 h or PV just after mixing with the gastric solution showed no reduction. These results suggest that the gastric solution inactivated PV at 37°C independently of hPVR expression in mice.
FIG. 1.
Inactivation of PV with a mouse gastric solution. (A) PV1(M)OM at 2 × 105 PFU/5 μl was incubated at 37°C or 0°C for the periods indicated with or without 45 μl of a gastric solution from PVRTg21 (Tg) or C57BL/6 (non-Tg) mice, and then the titer of virus was determined by a plaque assay. As a control, PV was incubated with 45 μl of saline. Three animals were used per group. (B) PV1(M)OM at 1 × 105 PFU/5 μl was incubated at 37°C or 0°C for the periods indicated with 45 μl of each solution. After the incubation, the titer of virus was determined by a plaque assay. Open bars, samples just after mixing; solid bars, incubation at 37°C for 4 h; shaded bars, incubation at 0°C for 4 h. Asterisks, nondetectable titers. ND, not detected.
Next, we investigated which factor influenced the inactivation. To examine the effect of low pH, 1 × 105 PFU of PV1(M)OM was incubated either with an HCl-containing solution at pH 1, pH 2, or pH 3, with saline (pH 7), or with the gastric solution, with or without NaHCO3, either unheated or heated to inactivate the enzymatic activities. The gastric solution had a pH of ∼1 without supplementation and a pH of 9 after it was mixed with NaHCO3. As shown in Fig. 1B, PV was inactivated by the pH 2 and pH 3 solutions, as well as by the gastric solution after incubation at 37°C, but not by saline (pH 7). With the pH 1 solution, PV was inactivated without incubation at 37°C or 0°C. These results suggest that a low pH can inactivate PV. To confirm the effect of the low pH, the gastric solution with NaHCO3 was incubated with PV. The pH 9 gastric solution did not inactivate PV even after incubation at 37°C. As a control, a saline solution with NaHCO3 (pH 9) was examined; it had no effect on the viral titer. These results suggest that the low pH of the gastric solution leads to inactivation of the virus. When HEPES was used instead of NaHCO3 to bring the viral solution to a pH of 9, the titer of virus was not decreased, either (data not shown). This result further supports the dependence of the PV-inactivating effect on a low pH. Finally, our experiment aimed to determine whether gastric enzymatic activities affect the infectivity of the virus. The enzymatic activities in the gastric solution were eliminated by heating at 95°C for 5 min. The heated gastric solution inactivated the virus after incubation at 37°C similarly to the unheated gastric solution without NaHCO3. This result suggests that the enzymatic activities in the gastric solution do not affect the infectivity of PV under the conditions used.
Efficient delivery of PV to the intestine after intragastric inoculation with a pH neutralizer.
To examine the survival rate of the virus at a low pH in the gastric environment in vivo, an assay of infectious PV was conducted after the virus was incubated in the stomach (Fig. 2A). Under anesthesia, the pylori were ligated and the mice were intragastrically inoculated with 7.8 × 107 PFU of PV/500 μl with or without 3% NaHCO3 by using a gastric tube. Five minutes after inoculation, the gastric contents were quickly recovered and the titer of the virus was determined by a plaque assay. When the mice were inoculated with PV without 3% NaHCO3, the titer was less than 0.1% of the original amount inoculated. On the other hand, when the mice were inoculated with PV together with 3% NaHCO3, around 20% of the inoculated virus was recovered from the stomach. These results suggest that PV can be inactivated by gastric contents in vivo similarly to the inactivation in vitro (Fig. 1). As shown in Fig. 1B, the virus with the gastric solution containing 3% NaHCO3 exhibited no loss of titer, whereas the virus inoculated intragastrically with 3% NaHCO3 showed an 80% loss of titer in vivo (Fig. 2A). The gastric solution used for the in vitro experiments had been diluted with saline, which may have resulted in the minor effect on the inactivation of PV.
FIG. 2.
Effect of passage through gastric contents on the virus titer after intragastric inoculation of mice with PV. (A) The effect of mouse gastric contents in the stomach on the retention of PV was examined. Under anesthesia, the pylorus was ligated and MPVRTg25 were intragastrically inoculated with 7.8 × 107 PFU of PV1(M)OM/500 μl with or without 3% NaHCO3 by using a gastric tube. Five minutes after inoculation, the gastric contents were quickly recovered and the virus was detected by a plaque assay. Each bar represents an individual mouse. The black area indicates the titer detected in the gastric contents, and the white area indicates the decrease in the inoculated titer. (B) The rate of recovery of the virus from the small intestines of MPVRTg25 was examined after intragastric inoculation with 7.8 × 107 PFU of PV1(M)OM/500 μl with or without 3% NaHCO3. Four hours after the inoculation, the contents of the small intestinal lumen were recovered and the virus was detected by a plaque assay. ND, not detected.
Next, we examined how much virus can reach the small intestine from the stomach after intragastric inoculation using a gastric tube. The rate of recovery of the virus from the small intestine was examined after intragastric inoculation of 7.8 × 107 PFU of PV with or without 3% NaHCO3 by using a gastric tube. Four hours after inoculation, the contents of the entire small intestine were recovered and the titer of the virus was determined by a plaque assay (Fig. 2B). For mice inoculated with PV without 3% NaHCO3, approximately 103 PFU/small intestinal lumen was recovered, whereas for mice inoculated with PV together with 3% NaHCO3, about 105 PFU/small intestinal lumen was recovered. These results indicate that NaHCO3 increased the recovery of the virus from the small intestinal lumen after intragastric inoculation with PV.
To know whether there is a reduction in the titer of the virus during the oral administration period (24 h), the stability of PV with 3% NaHCO3 after 24 h was examined. The pH of a 3% NaHCO3 solution was measured by pH test paper and determined to be 9. The titer was examined after incubation for 24 h at 0°C, room temperature, or 37°C (Fig. 3A and B). When PV1(M)OM was incubated with 3% NaHCO3 or with H2O, the titer did not decrease at 0°C or at room temperature. On the other hand, incubation of PV1(M)OM with 3% NaHCO3 at 37°C reduced the titer by approximately 5 orders of magnitude, whereas incubation with H2O at this temperature caused only about a 1-log-unit reduction (Fig. 3A). As for Sabin 1, when the virus was incubated at 0°C with 3% NaHCO3 or with H2O, no reduction in the titer was observed, whereas incubation at a higher temperature resulted in a reduction in the titer. Incubation of Sabin 1 with 3% NaHCO3 or with H2O at room temperature caused about a twofold reduction in the titer compared to that at 0°C. At 37°C, incubation of Sabin 1 with 3% NaHCO3 reduced the titer by roughly 6 orders of magnitude, whereas incubation with H2O caused only about a threefold reduction (Fig. 3B). These results suggest that incubation at room temperature for 24 h has only a minor effect on the titer and that incubation at 37°C for 24 h decreases the titer more severely, especially when the viral solution contains NaHCO3. The relatively high pH of the 3% NaHCO3 solution might have led to the instability of the viral RNA genome and virion particle. As for PV type 2 and 3 strains, incubation with 3% NaHCO3 had only a minor effect on Leon, but Lansing showed a ∼1-log-unit decrease in the titer after incubation with NaHCO3 even at room temperature for 24 h (data not shown). These results suggest that the stability of PV with NaHCO3 depends on the viral strain.
FIG. 3.
PV titer after incubation for 24 h with or without NaHCO3. A total of 1.5 × 108 PFU/ml of PV1(M)OM (A) or Sabin 1 (B) was incubated at the indicated temperatures for 24 h with (triangles) or without (circles) 3% NaHCO3. The titer of virus after incubation was determined by a plaque assay. rt, room temperature. ND, not detected.
Effects of IFN-α/β signaling on the cell permissivity of orally ingested PV.
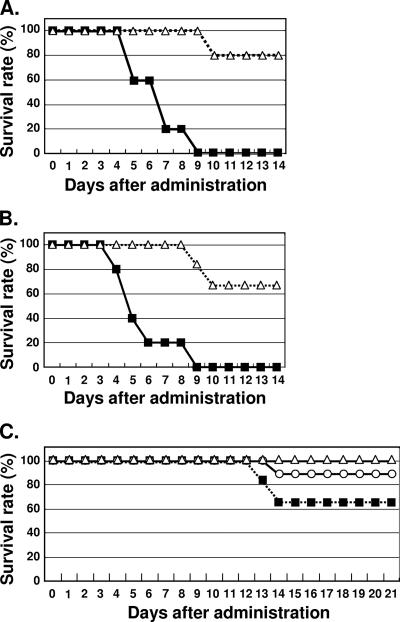
To test whether PV orally ingested with 3% NaHCO3 can cause paralysis in hPVR-Tg, PV with 3% NaHCO3 was orally administered to PVRTg21 or to PVRTg21/IfnarKO, which are deficient in Ifnar. To eliminate the possibility that the gastric tube might damage epithelia in the esophagus, 3 × 108 PFU of PV1(M)OM with 3% NaHCO3 was orally administered without using a gastric tube. Eighty percent of PVRTg21 survived, whereas all the PVRTg21/IfnarKO showed paralysis and died within 9 days of PV administration (Fig. 4A). These results suggest that PVRTg21/IfnarKO are more susceptible to orally administered PV1(M)OM than PVRTg21. The susceptibility of PVRTg21/IfnarKO is dependent on the titer of virus (data not shown).
FIG. 4.
Survival rates of mice after oral administration of PV. (A and C) PVRTg21/IfnarKO (solid squares) or PVRTg21 (open triangles) were orally administered 3 × 108 PFU/2 ml of PV1(M)OM (A) or Sabin 1 (C), and the rate of survival was determined. Alternatively, PVRTg21/IfnarKO were intravenously injected (open circles) with 1 × 105 PFU of Sabin 1/100 μl (C). Five (A), six (C) (oral administration), or nine (C) (intravenous injection) mice were observed for each group. (B) Similarly, MPVRTg25/IfnarKO (solid squares) or MPVRTg25 (open triangles) were orally administered 3 × 108 PFU of PV1(M)OM/2 ml. Five or six mice were observed per group. The rate of survival was determined.
To confirm the effect of IFN signaling on the pathogenicity of orally administered PV in mice, another Tg strain (MPVRTg25) was examined, because hPVR is apparently detected in the small intestine and liver of MPVRTg25 by Western blotting (43). MPVRTg25 and MPVRTg25/IfnarKO were orally administered PV with 3% NaHCO3 without using a gastric tube, and the clinical symptoms were observed (Fig. 4B). In agreement with the results obtained with PVRTg21 and PVRTg21/IfnarKO, all the MPVRTg25/IfnarKO died whereas only 33% of MPVRTg25 died. These results suggest that MPVRTg25/IfnarKO are more susceptible to oral administration of PV than MPVRTg25. These findings further support the notion that IFN signaling contributes to cell permissivity in mice orally administered PV. As for the clinical symptoms, MPVRTg25/IfnarKO tend to show hepatocirrhosis rather than paralysis, and it is highly possible that these mice died of a hepatic disorder. Nevertheless, one can observe paralysis after intracerebral inoculation of MPVRTg25/IfnarKO (data not shown). This result suggests that the virus can replicate in the CNS and cause paralysis even in MPVRTg25/IfnarKO.
When PVRTg21/IfnarKO or PVRTg21 were orally administered Sabin 1 with 3% NaHCO3 without use of a gastric tube, more than 30% of PVRTg21/IfnarKO showed paralysis and died, whereas all the PVRTg21 survived for 21 days (Fig. 4C). These results suggest that PVRTg21/IfnarKO were more susceptible to oral administration of Sabin 1 than PVRTg21. PVRTg21/IfnarKO also showed susceptibility to the type 2 strain Lansing or the type 3 strain Leon despite the fact that that Lansing was less pathogenic than PV1(M)OM or Leon (data not shown). Together with these results, our study strongly suggested that IFN signaling plays a key role in cell permissivity following oral administration of PV, although we cannot exclude the possibility that components of the innate immune system other than IFN-α/β affect the cell permissivity.
Time course of the replication of PV in tissues after oral administration.
To assess the ability of PV to replicate in different tissues after oral administration, the titers of the virus in the small intestine, colon, nasopharynx-associated lymphoid tissue (NALT), esophagus, spinal cord, and plasma were determined at 1, 2, and 3 days after administration to mice. PV was orally administered to PVRTg21, PVRTg21/IfnarKO, MPVRTg25, MPVRTg25/IfnarKO, C57BL/6, and C57BL/6/IfnarKO, and the tissues were excised each day after administration. The tissues were then homogenized, and the titers of the virus in the solutions were determined by a plaque assay (Fig. 5A). In all the tissues tested, titers were always higher in Ifnar knockout hPVR-Tg than in Ifnar-expressing hPVR-Tg. For instance, the titers were higher in PVRTg21/IfnarKO than in PVRTg21, and similarly, they were higher in MPVRTg25/IfnarKO than in MPVRTg25 (Fig. 5Aa to c, e, and f). As for C57BL/6/IfnarKO and C57BL/6 mice, the titers in the upper small intestine were negligible (Fig. 5Aa). These results suggest that PV can replicate in all of the tissues examined more efficiently in Ifnar knockout mice than in Ifnar-expressing mice.
FIG. 5.
Titers of PV recovered in tissues after oral administration of PV. (A) Virus was extracted from tissues of mice (PVRTg21 [open triangles], PVRTg21/IfnarKO [solid triangles], MPVRTg25 [open circles], MPVRTg25/IfnarKO [solid circles], C57BL/6 [open squares], and C57BL/6/IfnarKO [solid squares]) 1, 2, and 3 days after the administration of 3 × 108 PFU of PV1(M)OM/2 ml. The vertical axis shows the amount (PFU) of PV detected in tissues by the plaque assay. USI, upper small intestine; LSI, lower small intestine. (B) Virus was extracted from each tissue of PVRTg21/IfnarKO every 24 h after oral administration of 3 × 108 PFU of PV1(M)OM/2 ml (a to f) or intravenous injection of 1 × 105 PFU of PV1(M)OM/100 μl (g to l). Each point indicates one mouse. ND, not detected.
As for plasma, the virus caused viremia from the second day in PVRTg21/IfnarKO, MPVRTg25, and MPVRTg25/IfnarKO (except for one animal that showed slight viremia on the first day) (Fig. 5Ad). The results imply that the virus leaks into the blood after replicating in tissues, probably the alimentary tract, because little virus was detected in plasma on the first day.
We next investigated the chronological titer of the virus in different tissues after an oral or systemic challenge (Fig. 5B). When PVRTg21/IfnarKO were intravenously inoculated with PV1(M)OM, the virus had started replicating extensively in all tissues examined on the third day (Fig. 5Bg to l). Almost all the mice injected intravenously with the virus developed paralysis and died on the fifth day (data not shown). Following oral administration of PV1(M)OM to PVRTg21/IfnarKO, all the tissues examined showed a burst of proliferation of the virus on the fifth day (Fig. 5Ba to f), and the mice began to die on the fifth day (Fig. 4A). These findings indicate that the burst of replication after oral administration may be due to the virus that appeared on the second day in the bloodstream, because such a burst took 3 days after the intravenous injection. It seems feasible that the paralysis after oral administration of the virus is mainly due to the circulating virus that invaded from the alimentary tract.
Inoculated PV was incorporated into mouse small intestinal epithelia.
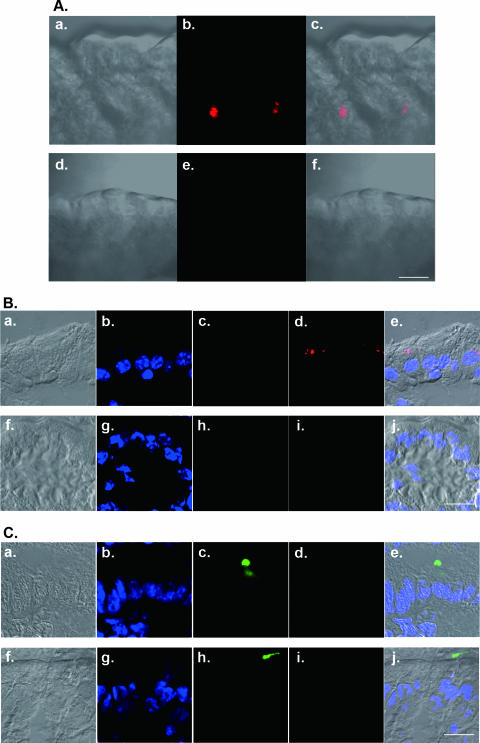
To examine which kind of cells in the alimentary tract incorporate the virus, fluorescently labeled PV was injected into the ligated small intestine in MPVRTg25/IfnarKO. One hour after the injection, the ligated tissue was subjected to confocal laser scanning microscopic analysis. As shown in Fig. 6Ab and c, the virus was detected inside the microvillus, whereas no fluorescence was detected in the small intestine without the injection (Fig. 6Ae and f). When the intestine was observed after frozen sectioning, the virus was detected in the cytoplasm of the epithelial cells in the microvilli (Fig. 6Bd and e). No fluorescence was detected in the small intestine without injection of the virus (Fig. 6Bi and j). These results suggest that the virus inside the cavity of the small intestine can be incorporated into the epithelial cells. It is highly possible that the incorporated virus starts to replicate in these epithelial cells. To further clarify which kinds of cells incorporate the virus from the intestinal cavity, fluorescently labeled UEA-1 (specific for α-l-fucose residues) was used, since UEA-1 has been shown to possess high affinity for selected intestinal epithelial cells, including mouse microfold (M) cells and goblet cells (4, 17). As shown in Fig. 6C, PV was not detected in the UEA-1-positive fraction of epithelial cells. On the other hand, the cells that contained PV were the UEA-1-negative fraction in the epithelia (Fig. 6B). It has been reported that some microorganisms, such as Salmonella enterica serovar Typhimurium and Yersinia pseudotuberculosis, can be efficiently incorporated into M cells under similar experimental conditions (15). These results suggest that in the IFN-α/β-free environment of the intestinal epithelium, PV is incorporated into the UEA-1-negative fraction of epithelial cells, although we cannot exclude the possibility that M cells are involved in the dissemination of the virus and the subsequent infection process.
FIG. 6.
Fluorescently labeled PV is detected in intestinal epithelia. Alexa Fluor 555-labeled PV1(M)OM (1.5 × 107 PFU/10 μl) was injected into the ligated small intestines of MPVRTg25/IfnarKO (top row of images in each panel). PV-negative controls were also used (bottom row in each panel). One hour after injection, the ligated portion was excised and subjected to confocal laser scanning microscopy. Intestines were observed without (A) or with (B and C) fixation and frozen sectioning. (B) UEA-1-negative epithelia; (C) epithelia that contain UEA-1-positive cells. Red, Alexa Fluor 555-labeled PV1(M)OM (Ab, c, e, and f; Bd, e, i, and j; Cd, e, i, and j); blue, nuclei (Bb, e, g, and j; Cb, e, g, and j); green, fluorescein isothiocyanate-labeled UEA-1 (Bc, e, h, and j; Cc, e, h, and j). Aa and d, Ba and f, and Ca and f show bright-field images only. Ac and f, Be and j, and Ce and j show merged images of bright-field and fluorescence micrographs. Bars, 100 μm.
Oral administration of attenuated PV did not effectively generate neutralizing antibodies in hPVR-Tg/IfnarKO.
Inasmuch as orally administered PV is incorporated into the intestinal epithelium and starts replicating in hPVR-Tg/IfnarKO, it is important to examine whether oral administration of attenuated PV can induce the production of neutralizing antibodies in mice. To this end, attenuated PV was orally administered to PVRTg21/IfnarKO or PVRTg21. Serum was collected 21 days after virus administration, and the neutralizing activity in the serum was assayed. In the case of PVRTg21/IfnarKO, all the mice showed neutralizing activity after intravenous inoculation of 105 PFU of Sabin 1 whereas no mouse showed neutralizing activity after oral administration of 3 × 108 PFU of the strain or DMEM (Table 1), although the virus was detected in the intestine until 4 days after oral administration of Sabin 1 (data not shown). As for PVRTg21, no mouse showed neutralizing activity after oral administration of 3 × 108 PFU of Sabin 1. Similar results were obtained for MPVRTg25/IfnarKO after oral administration of the strain (data not shown). These results suggest that oral administration of Sabin 1 to PVRTg21/IfnarKO or MPVRTg25/IfnarKO was ineffective at raising the neutralizing activity.
TABLE 1.
PV-neutralizing activity in serum after oral administration of Sabin 1
| Mouse strain | Substance | Route of administration | Amt of immunizing virus (PFU/mouse) | Neutralizing activitya |
|---|---|---|---|---|
| PVRTg21/ IfnarKO | DMEM | Oral | 0 | 0/6 |
| Sabin 1 | Oral | 3 × 108 | 0/4 | |
| Sabin 1 | Intravenous | 1 × 105 | 8/8 | |
| PVRTg21 | Sabin 1 | Oral | 3 × 108 | 0/6 |
Expressed as the number of mice whose serum showed neutralizing activity (≥16)/number of mice examined.
DISCUSSION
Some of the recent outbreaks in areas certified as being clear of polio were caused by a circulating vaccine-derived PV that had mutated from the oral polio vaccine used to prevent polio (18). This suggests to us a need to develop new polio vaccines or antipolio drugs for the control of polio outbreaks. For that purpose, it is necessary to establish a useful animal model that mimics the natural infection route and subsequent disease development in humans in order to evaluate candidate vaccines or drugs.
A paper about the effects of pH on PV infectivity (40) indicates that PV can be easily inactivated at pH 3.0 or 9.0 at 30°C, but it depends on the buffers. This means that, at least at pH 3.0 or 9.0, the stability of viral infectivity is determined by the stability of the virions in particular buffers.
There is a discrepancy in the data obtained at pH 1 between the HCl solution and the gastric solution (Fig. 1B). The fact that the pH was measured using pH test paper and we cannot know the value precisely might explain the discrepancy, especially at the extremely low pH.
The present study has shown that IFNAR plays an important role in the infection and multiplication of orally administered PV in the small intestine of hPVR-Tg (Fig. 4 and 5A). The deletion of IFNAR resulted in successful infection by oral PV via the intestinal epithelium and the subsequent development of clinical symptoms. Viremia seems to be essential for the symptoms to appear (Fig. 5B), and a histopathology for similar symptoms caused by artificial viremia (intravenous inoculation) in hPVR-Tg/IfnarKO has been reported (13). We thus established an oral administration system using hPVR-Tg/IfnarKO, with which one can assess the 100% lethal doses of PV strains. This is the first in vivo system in which all the animals showed paralysis after oral administration of PV.
From the results presented in Fig. 5B, the orally administered virus disseminates mainly through the bloodstream in mice, although other, minor routes might be involved. It is possible, for example, that a neural pathway exists from the alimentary tract to the CNS through the vagus nerve or from the skeletal muscle to the CNS through the peripheral nerve. After PVRTg21/IfnarKO were orally administered 3 × 108 PFU of Sabin 1, low titers (from 4 × 101 PFU/plasma to 8 × 102 PFU/plasma) of virus were detected in the plasma for 2 of 3 mice 3 days after administration and for 1 of 3 mice 4 days after administration. In spite of the ineffective serum conversion (Table 1), some lethal infection occurs after oral administration of Sabin 1 in PVRTg21/IfnarKO (Fig. 4C). These results imply that the neural pathway from the alimentary tract to the CNS might contribute to death after oral administration of Sabin 1 in PVRTg21/IfnarKO. After oral administration, the titer of virus in skeletal muscle did not rise until the virus started replicating efficiently in all the tissues examined (data not shown). This result implies that the neural pathway from skeletal muscle to the CNS is not essential, at least in this system. Nevertheless, we do not know which pathway has an essential role in causing paralysis and death.
It is possible that the virus was incorporated accidentally via the intranasal pathway after oral administration. In our experiments, when 1.5 × 108 PFU/ml of the Mahoney strain was orally administered to PVRTg21, the mice showed hardly any signs of paralysis. This concentration was higher than 106 PFU/20 μl, which was enough to cause death among 60% of intranasally inoculated PVRTg21 (29). Furthermore, the distribution of the virus after oral administration differs from that after intranasal inoculation. From these results, it is unlikely that orally administered virus enters the intranasal pathway.
We have shown previously that hPVR is expressed in the small intestines of MPVRTg25 but is not detected in those of PVRTg21 by Western blotting (43). As for immunohistochemistry, hPVR expression was barely observed in the intestinal epithelium and was not detected in germinal centers within Peyer's patches in PVRTg21 (14), and an assertive hPVR antigen was not detected in small intestinal epithelia in PVRTg21 or MPVRTg25 (M. Takano-Maruyama and H. Ohno, personal communication). These results suggest that hPVR is not expressed at high levels in the small intestines of PVRTg21, MPVRTg25, or the Ifnar knockout versions of these mice, although it is possible that the levels of hPVR expression on the intestinal epithelia differ among these mice. Incorporated fluorescently labeled virus was observed in the intestines of MPVRTg25/IfnarKO but not PVRTg21/IfnarKO or C57BL/6 mice (data not shown). This might be due to the level of hPVR expression on the apical side of the intestinal epithelia. Despite the fact that PVRTg21 express little if any hPVR in the small intestine, the titers of virus in the upper small intestines of PVRTg21 and PVRTg21/IfnarKO 1 day after administration were ∼102-fold higher than those in C57BL/6 mice and C57BL/6/IfnarKO, respectively (Fig. 5A), and PVRTg21/IfnarKO showed susceptibility to oral administration of PV (Fig. 4A and C and 5). These results suggest that hPVR expressed in the small intestines of PVRTg21/IfnarKO contributes to cell susceptibility to orally administered PV.
We observed hepatocirrhosis in MPVRTg25/IfnarKO after oral administration of PV1(M)OM despite the fact that MPVRTg25 do not show hepatocirrhosis. These results suggest that MPVRTg25/IfnarKO show irregular tissue tropism of the virus compared to MPVRTg25, which have a native immune system. A previous paper has reported that viral antigen-positive cells were detected in the liver 1 day after intravenous inoculation of 2 × 107 PFU of PV1(M)OM into MPVRTg25/IfnarKO (13). Moreover, a high titer of the virus was recovered 2 days after oral administration of 3 × 108 PFU of PV1(M)OM to MPVRTg25/IfnarKO (data not shown). It seems that this occurred because hPVR in the liver causes the virus to replicate in MPVRTg25/IfnarKO mice. Indeed, we have reported previously that the liver in MPVRTg25 expresses hPVR (43). Moreover, the virus in the bloodstream can easily access the liver (1). Considering these results, it is feasible that the viremia led to a liver infection that then caused a secondary viremia, leading to invasion of the CNS in MPVRTg25/IfnarKO. As for PVRTg21/IfnarKO, it has been reported that the disruption of IFNAR enables the virus to replicate in nonneuronal tissues, although both PVRTg21 and PVRTg21/IfnarKO developed paralysis by similar points in time after intravenous inoculation (13). All together, the oral infection system using hPVR-Tg/IfnarKO does not serve as an adequate animal model for analyzing virus tissue tropism in the whole body, although the clinical symptoms seen in PVRTg21/IfnarKO were similar to those in PVRTg21. This notion is also supported by the fact that healthy humans are highly susceptible to poliovirus infection in spite of a robust innate immune response. Nevertheless, this oral administration system might be applicable to the study of initial infection events in vivo. It is also worth elucidating the role of the IFNAR-related signaling cascade in the infection of intestinal epithelial cells.
We succeeded in detecting the fluorescently labeled virus in the small intestine 1 h after its injection (Fig. 6) but failed to detect the viral antigen 2 days after oral administration of the virus using immunohistochemistry (data not shown). It is difficult to detect antigens in the intestine because of high background levels and the quick turnover of infected cells. It is possible that the virus does not replicate prominently in the intestinal epithelia or that the infected cells tend to drop out easily from the epithelial layer. Although the titers of virus in other alimentary tissues were relatively low after oral administration of the virus (Fig. 5A) and the viral antigen was not detected in the NALT, esophagus, or colon 2 days after oral administration (data not shown), we cannot exclude the possibility that PV replicates in the alimentary tract early in the course of infection. Alternatively, the fluorescence observed could have derived from inactive PV taken up by cells during a nonproductive infection.
The mechanism for oral infection with PV in humans has not been made clear, though it is possible that PV can replicate in and/or permeate M cells or lymphatic tissues in humans, because attenuated PV vaccine strains can readily generate neutralizing antibodies. In the present study, oral administration of Sabin 1 did not lead to the generation of neutralizing activity in the serum of PVRTg21/IfnarKO (Table 1) and MPVRTg25/IfnarKO (data not shown) or the binding activity of immunoglobulin A's in feces (data not shown), despite the fact that the virus proliferated in the upper small intestine (data not shown). Our results also showed that PV was not preferentially detected in the UEA-1 fraction of epithelial cells, which contains M cells, in hPVR-Tg/IfnarKO (Fig. 6C), and the virus did not proliferate efficiently in NALT, a lymphatic tissue (Fig. 5Ae and Be). These results may correlate with the difficulty of raising the neutralizing activity after oral administration of the virus in hPVR-Tg/IfnarKO. However, it should be emphasized that a lack of IFNAR hampered enhancement of the antibody-evoked response (23). Thus, the phenomena we observed might be due to the lack of IFNAR. Although we still could not reconcile the discrepancy between humans and the murine model in the exact site of PV invasion in the intestinal tract, the present study offers a new opportunity to address the issue, since hPVR-Tg/IfnarKO are susceptible to orally administered PV for the initiation of infection and subsequent development of disease symptoms. And it is probable that the expression of hPVR in different intestinal cells is important in determining the heightened permissiveness of oral PV administration in humans compared to rodents. PV is highly successful at infection of the alimentary tracts of humans, and intestinal secretion of the virus can persist for months, even in healthy individuals, regardless of age. This means that the virus infects and replicates in some cells or tissues in the human gastrointestinal tract in spite of a possible early innate immune response. However, it is also possible that the innate immune response of humans is weaker than that of rodents.
The presumed barriers to orally ingested PV are shown in Fig. 7. First, the ingested virus enters the stomach and experiences a low-pH environment (the first barrier). If the virus overcomes this, it has to find the appropriate receptor in order to enter the intestinal cells (the second barrier, cell susceptibility). Then the virus can start replicating in the cells depending on the lack of an IFN system (the third barrier, cell permissivity). Finally, the virus probably invades the bloodstream. In this report, we clarified that the low pH of the gastric environment inactivates PV and decreases the titer of virus that reaches the intestine, and the lack of an IFN system allows the virus to replicate in the intestine more efficiently. These three barriers may serve to protect individuals from viremia.
Acknowledgments
We are grateful to T. Matano for suggestions and discussions. We also thank A. Ohmura for help in breeding the mice and E. Suzuki for help in preparing the manuscript.
This work was supported in part by Grants-in-Aid for Advanced Medical Science Research, a Grant-in-Aid for Scientific Research on Priority Areas by the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a grant from the Ministry of Health and Welfare of Japan.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Aoki, J. 1994. Tissue tropism and species specificity of poliovirus infection. Tanpakushitsu Kakusan Koso 39:2081-2089. [PubMed] [Google Scholar]
- 2.Bodian, D. 1955. Emerging concept of poliomyelitis infection. Science 122:105-108. [DOI] [PubMed] [Google Scholar]
- 3.Chebath, J., P. Benech, M. Revel, and M. Vigneron. 1987. Constitutive expression of (2′-5′) oligo A synthetase confers resistance to picornavirus infection. Nature 330:587-588. [DOI] [PubMed] [Google Scholar]
- 4.Clark, M. A., M. A. Jepson, N. L. Simmons, T. A. Booth, and B. H. Hirst. 1993. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cytochem. 41:1679-1687. [DOI] [PubMed] [Google Scholar]
- 5.Coccia, E. M., G. Romeo, A. Nissim, G. Marziali, R. Albertini, E. Affabris, A. Battistini, G. Fiorucci, R. Orsatti, G. B. Rossi, et al. 1990. A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology 179:228-233. [DOI] [PubMed] [Google Scholar]
- 6.Fiette, L., C. Aubert, U. Muller, S. Huang, M. Aguet, M. Brahic, and J. F. Bureau. 1995. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J. Exp. Med. 181:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flint, S. J., R. M. Enquist, V. R. Racaniello, and A. M. Skalka. 2003. Principles of virology: molecular biology, pathogenesis, and control of animal viruses. ASM Press, Washington, DC.
- 8.Garcia-Sastre, A., R. K. Durbin, H. Zheng, P. Palese, R. Gertner, D. E. Levy, and J. E. Durbin. 1998. The role of interferon in influenza virus tissue tropism. J. Virol. 72:8550-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier, M., and E. Wimmer. 1998. Mechanism of injury-provoked poliomyelitis. J. Virol. 72:5056-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horie, H., S. Koike, T. Kurata, Y. Sato-Yoshida, I. Ise, Y. Ota, S. Abe, K. Hioki, H. Kato, C. Taya, T. Nomura, S. Hashizume, H. Yonekawa, and A. Nomoto. 1994. Transgenic mice carrying the human poliovirus receptor: new animal models for study of poliovirus neurovirulence. J. Virol. 68:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe, H., and D. Bodian. 1942. Neuronal mechanisms in poliomyelitis. Commonwealth Fund, New York, NY; Humphrey Milford, Oxford University Press, London, England.
- 12.Hsiung, G. D., F. L. Black, and J. R. Henderson. 1964. Susceptibility of primates to viruses in relation to taxonomic classification, p. 1-23. In J. Buettner-Janusch (ed.), Evolutionary and genetic biology of primates, vol. II. Academic Press, New York, NY. [Google Scholar]
- 13.Ida-Hosonuma, M., T. Iwasaki, T. Yoshikawa, N. Nagata, Y. Sato, T. Sata, M. Yoneyama, T. Fujita, C. Taya, H. Yonekawa, and S. Koike. 2005. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J. Virol. 79:4460-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki, A., R. Welker, S. Mueller, M. Linehan, A. Nomoto, and E. Wimmer. 2002. Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 186:585-592. [DOI] [PubMed] [Google Scholar]
- 15.Jang, M. H., M. N. Kweon, K. Iwatani, M. Yamamoto, K. Terahara, C. Sasakawa, T. Suzuki, T. Nochi, Y. Yokota, P. D. Rennert, T. Hiroi, H. Tamagawa, H. Iijima, J. Kunisawa, Y. Yuki, and H. Kiyono. 2004. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 101:6110-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajigaya, S., H. Arakawa, S. Kuge, T. Koi, N. Imura, and A. Nomoto. 1985. Isolation and characterization of defective-interfering particles of poliovirus Sabin 1 strain. Virology 142:307-316. [DOI] [PubMed] [Google Scholar]
- 17.Kandori, H., K. Hirayama, M. Takeda, and K. Doi. 1996. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp. Anim. 45:155-160. [DOI] [PubMed] [Google Scholar]
- 18.Kew, O. M., R. W. Sutter, E. M. de Gourville, W. R. Dowdle, and M. A. Pallansch. 2005. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 59:587-635. [DOI] [PubMed] [Google Scholar]
- 19.Kohara, M., S. Abe, T. Komatsu, K. Tago, M. Arita, and A. Nomoto. 1988. A recombinant virus between the Sabin 1 and Sabin 3 vaccine strains of poliovirus as a possible candidate for a new type 3 poliovirus live vaccine strain. J. Virol. 62:2828-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koike, S., J. Aoki, and A. Nomoto. 1994. Transgenic mice for the study of poliovirus pathogenicity, p. 463-480. In E. Wimmer and R. Weiss (ed.), Cellular receptors for animal viruses. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 21.Koike, S., C. Taya, J. Aoki, Y. Matsuda, I. Ise, H. Takeda, T. Matsuzaki, H. Amanuma, H. Yonekawa, and A. Nomoto. 1994. Characterization of three different transgenic mouse lines that carry human poliovirus receptor gene—influence of the transgene expression on pathogenesis. Arch. Virol. 139:351-363. [DOI] [PubMed] [Google Scholar]
- 22.Koike, S., C. Taya, T. Kurata, S. Abe, I. Ise, H. Yonekawa, and A. Nomoto. 1991. Transgenic mice susceptible to poliovirus. Proc. Natl. Acad. Sci. USA 88:951-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 24.Meurs, E. F., Y. Watanabe, S. Kadereit, G. N. Barber, M. G. Katze, K. Chong, B. R. Williams, and A. G. Hovanessian. 1992. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J. Virol. 66:5805-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison, M. E., and V. R. Racaniello. 1992. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J. Virol. 66:2807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrkic, B., J. Pavlovic, T. Rulicke, P. Volpe, C. J. Buchholz, D. Hourcade, J. P. Atkinson, A. Aguzzi, and R. Cattaneo. 1998. Measles virus spread and pathogenesis in genetically modified mice. J. Virol. 72:7420-7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 28.Munoz, A., and L. Carrasco. 1984. Action of human lymphoblastoid interferon on HeLa cells infected with RNA-containing animal viruses. J. Gen. Virol. 65:377-390. [DOI] [PubMed] [Google Scholar]
- 29.Nagata, N., T. Iwasaki, Y. Ami, Y. Sato, I. Hatano, A. Harashima, Y. Suzaki, T. Yoshii, T. Hashikawa, T. Sata, Y. Horiuchi, S. Koike, T. Kurata, and A. Nomoto. 2004. A poliomyelitis model through mucosal infection in transgenic mice bearing human poliovirus receptor, TgPVR21. Virology 321:87-100. [DOI] [PubMed] [Google Scholar]
- 30.Nathanson, N., and A. D. Langmuir. 1963. The Cutter incident. Poliomyelitis following formaldehyde-inactivated poliovirus vaccination in the United States during the spring of 1955. III. Comparison of the clinical character of vaccinated and contact cases occurring after use of high rate lots of Cutter vaccine. Am. J. Hyg. 78:61-81. [DOI] [PubMed] [Google Scholar]
- 31.Ohka, S., W. X. Yang, E. Terada, K. Iwasaki, and A. Nomoto. 1998. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology 250:67-75. [DOI] [PubMed] [Google Scholar]
- 32.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 33.Ren, R., and V. R. Racaniello. 1992. Human poliovirus receptor gene expression and poliovirus tissue tropism in transgenic mice. J. Virol. 66:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren, R., and V. R. Racaniello. 1992. Poliovirus spreads from muscle to the central nervous system by neural pathways. J. Infect. Dis. 166:747-752. [DOI] [PubMed] [Google Scholar]
- 35.Ren, R. B., F. Costantini, E. J. Gorgacz, J. J. Lee, and V. R. Racaniello. 1990. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell 63:353-362. [DOI] [PubMed] [Google Scholar]
- 36.Ryman, K. D., W. B. Klimstra, K. B. Nguyen, C. A. Biron, and R. E. Johnston. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabin, A. B. 1965. Oral poliovirus vaccine: history of its development and prospects for eradication of poliomyelitis. JAMA 194:872-876. [DOI] [PubMed] [Google Scholar]
- 38.Sabin, A. B. 1956. Pathogenesis of poliomyelitis: reappraisal in the light of new data. Science 123:1151-1157. [DOI] [PubMed] [Google Scholar]
- 39.Sabin, A. B., and L. R. Boulger. 1973. History of Sabin attenuated poliovirus oral live vaccine strains. J. Biol. Stand. 1:115-118. [Google Scholar]
- 40.Salo, R. J., and D. O. Cliver. 1976. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch. Virol. 52:269-282. [DOI] [PubMed] [Google Scholar]
- 41.Shiroki, K., T. Ishii, T. Aoki, M. Kobashi, S. Ohka, and A. Nomoto. 1995. A new cis-acting element for RNA replication within the 5′ noncoding region of poliovirus type 1 RNA. J. Virol. 69:6825-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessely, R., K. Klingel, K. U. Knowlton, and R. Kandolf. 2001. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation 103:756-761. [DOI] [PubMed] [Google Scholar]
- 43.Yanagiya, A., S. Ohka, N. Hashida, M. Okamura, C. Taya, N. Kamoshita, K. Iwasaki, Y. Sasaki, H. Yonekawa, and A. Nomoto. 2003. Tissue-specific replicating capacity of a chimeric poliovirus that carries the internal ribosome entry site of hepatitis C virus in a new mouse model transgenic for the human poliovirus receptor. J. Virol. 77:10479-10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, W. X., T. Terasaki, K. Shiroki, S. Ohka, J. Aoki, S. Tanabe, T. Nomura, E. Terada, Y. Sugiyama, and A. Nomoto. 1997. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology 229:421-428. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, S., and V. R. Racaniello. 1997. Expression of the poliovirus receptor in intestinal epithelial cells is not sufficient to permit poliovirus replication in the mouse gut. J. Virol. 71:4915-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]