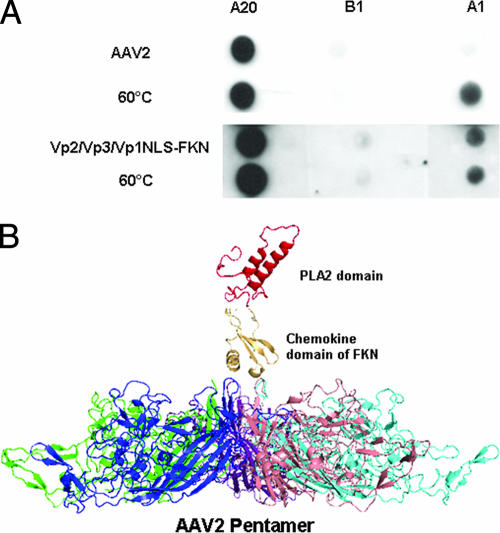
FIG. 3.
(A) Native dot blot Western analysis of purified AAV2 and Vp2/Vp3/Vp1NLSFKN. A20 (MAb to intact AAV2 virions), B1 (MAb to individual capsid proteins), and A1 (MAb to the unique N terminus of VP1) were used to assess the surface exposure of wt VP1 for AAV2 when heated to 60°C and Vp1NLSFKN when incorporated into Vp2/Vp3-only particles at room temperature. (B) Schematic representation of the surface-exposed domains of the Vp1 fusion proteins. This is not a predicted structure but was compiled to provide a visualization of the possible juxtaposition of the FKN and PLA2 protein regions above the AAV2 fivefold pore region. It was generated from the crystal structure of the AAV2 Vp3 protein (Protein Data Bank [PDB] identifier 1LP3), the structure of the chemokine domain of FKN (PDB identifier 1F2L), and a homologous model of the AAV2 PLA2 domain generated by sequence comparison to the PLA2 domain structure of bee venom using the SWISS MODEL algorithm (33a; http://www.expasy.org). The FKN and PLA2 domain structures were visually “docked” above the fivefold pore in the AAV2 Vp3 pentamer in the PyMol program (W. L. DeLano, The PyMol molecular graphics system, DeLano Scientific, San Carlos, CA, 2002).