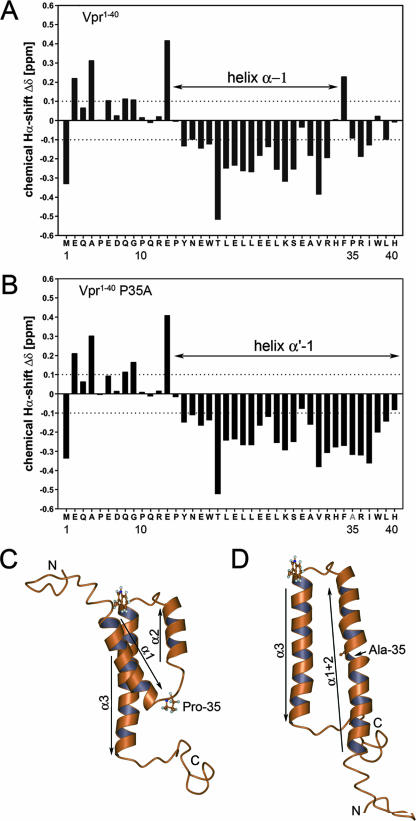
FIG. 4.
(A and B) 1H chemical shift differences (Δδ) of the α-protons between the experimentally determined values and those for residues in a random coil for sVpr1-40 (A) and sVpr1-40 P35A (B). (C) Experimental 3-dimensional structure of Vpr (available at http://www.pdb.org under PDB ID 1M8L and published in reference 16) showing the relative positions of the three helices and Pro-35. (D) Calculated conformation of the P35A mutant in which helix 1 (α1) has merged with helix 2 (α2) to give an antiparallel arrangement of helices. This was modeled by moving helix 1 and folding the interhelical residues, between helices 1 and 2, into a helical conformation to form a merged, extended structure, termed helix 1+2 (α1+2). The relative positions of the original helices 2 and 3 were unaltered in this conformation.