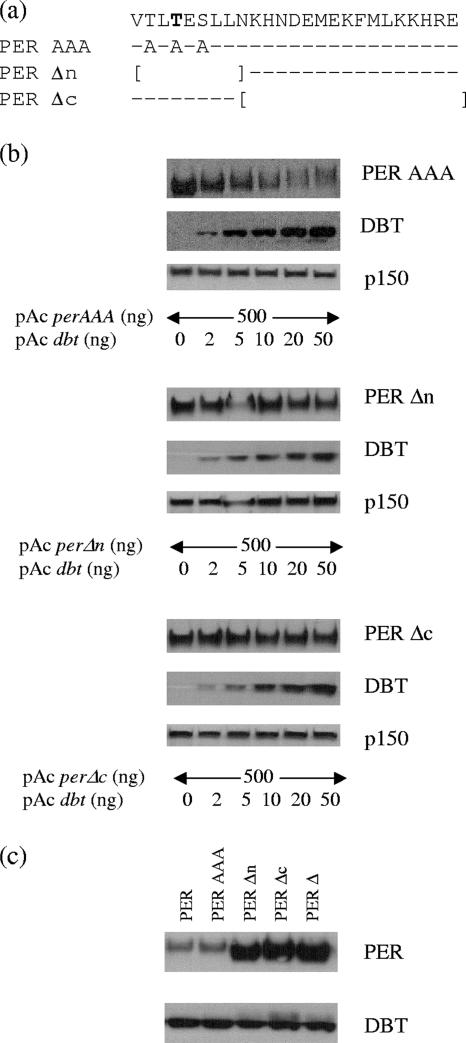
FIG. 2.
This conserved region likely functions as a protein-protein interaction domain rather than as a required initial phosphorylation site. (a) A representation of three additional mutants of otherwise wild-type PER protein, with PER-AAA having all possible phosphorylation sites mutated and replaced by alanine and PERΔn and PERΔc having smaller internal deletions (indicated by brackets). The conserved sequence is on the top line, dashes indicate no change in amino acids, and boldface indicates the conserved threonine found in Drosophila and mammalian PER proteins. (b) Western blot analysis of these three mutants from S2 cells overexpressing PER and increasing amounts of DBT. (c) Comparison of electrophoretic mobilities of wild-type PER and various mutants.