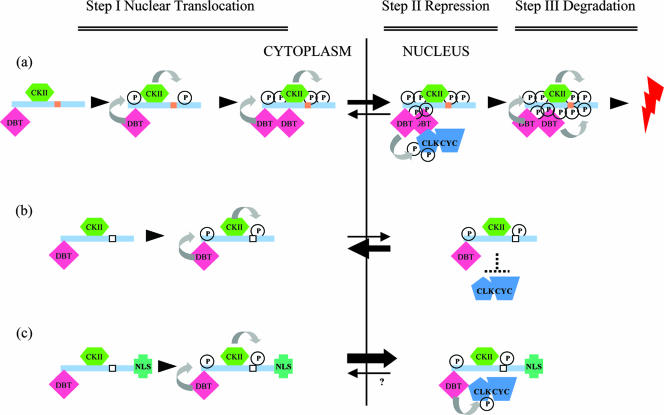
FIG. 7.
Model. (a) We propose that the conserved domain (brown rectangle) is involved in progressive phosphorylation. In the first step (step I), newly synthesized PER is phosphorylated by DBT and CKII; however, this progressive phosphorylation is dependent on the conserved domain (see the text for more detail). This domain could enhance phosphorylation either by interacting with kinases itself or by helping to recruit other kinases to PER (DBT and CKII included). In any case, proper phosphorylation leads to PER nuclear translocation. In the second step within the nucleus (step II), PER represses CLK-CYC transcriptional activation in association with DBT, as recently suggested (25, 55), and perhaps with CKII. In the third step (step III), phosphorylation by DBT and CKII promotes PER degradation. (b) Removal of the conserved domain (empty rectangle) weakens the kinase-PER interactions, disables progressive phosphorylation, inhibits PER nuclear localization, and results in a large reduction in repression activity. (c) Addition of an NLS increases PERΔ repression activity, suggesting that PERΔ is an active repressor and that the reduction in PERΔ repression activity is primarily due to a defect in nuclear localization. Curved arrows indicate progressive phosphorylation, whereas forward and reverse black arrows indicate nuclear entry and export, respectively. Although the rate of nuclear export of PERΔ-NLS is uncertain, it is apparently negligible relative to that of nuclear entry.