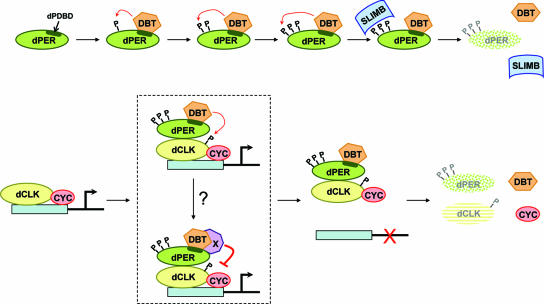
FIG. 8.
Model for the multiple roles of DBT in regulating the levels and activities of dPER and dCLK. (A) Role for DBT in the hyperphosphorylation and degradation of dPER. DBT stably associates with dPER via the dPDBD (dark green stripe within the dPER oval), which promotes the progressive phosphorylation (denoted by the letter “P”) of dPER by DBT and/or other kinases (for simplicity, TIM and other possible factors are not shown). SLIMB preferentially interacts with hyperphosphorylated isoforms of dPER (represented by three P's), targeting them to the 26S proteasome for rapid degradation. (B) In the absence of nucleus-localized dPER, the dCLK-CYC transcription factor binds E-box containing regulatory elements and drives expression of clock and output genes. Various phosphoisoforms of dCLK are observed throughout a daily cycle (hypophosphorylated, intermediately phosphorylated, and hyperphosphorylated), but for simplicity the hypophosphorylated and intermediately phosphorylated variants are represented by the same object (yellow oval with no phosphates), whereas only one phosphate is used to represent the DBT-dependent hyperphosphorylated species of dCLK. Eventually, a heteromeric complex containing DBT bound to dPER enters the nucleus, whereby dPER interacts with dCLK. The interaction of dPER with dCLK enhances the ability of DBT to phosphorylate dCLK, which stimulates its rapid degradation and might directly lead to its dissociation from DNA. It is also possible that additional steps, indicated by the binding of factor “X,” are required to block dCLK-CYC transactivation activity and release from DNA. Factor “X” might directly bind to the dPDBD or interact with DBT (as shown). DBT-dependent hyperphosphorylated dCLK is subject to enhanced degradation, although the putative F-box mediating this targeting is not known. It is also not clear if dissociation from DNA is required for the rapid degradation of hyperphosphorylated dCLK.