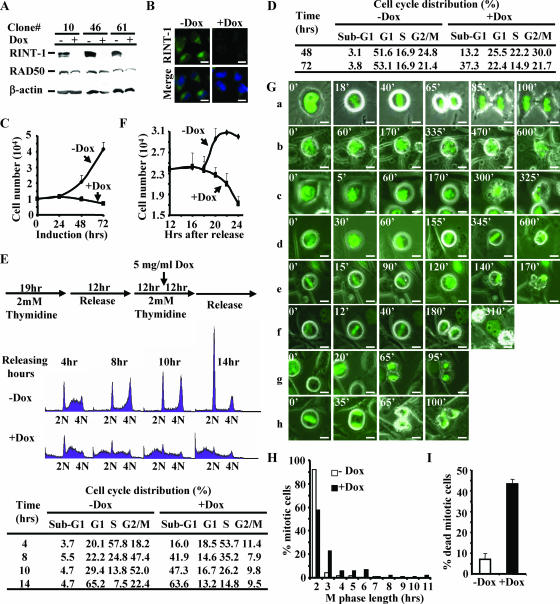
FIG. 4.
Depletion of RINT-1 leads to a prolonged M phase and severe mitotic cell death. (A) Characterization of three HeLa cell clones with inducible expression of RINT-1 RNAi. Cells were treated with (+) or without (−) 5 μg of doxycycline/ml for 48 h. The cell lysates were analyzed by Western blotting and probed with antibodies against RINT-1, RAD50, and β-actin. The expression of RINT-1 protein was diminished when RINT-1 RNAi was induced with doxycycline. RAD50 and β-actin served as controls. (B) Confirmation of the depletion of RINT-1 protein in HeLa-RINT-1i cells upon addition of 5 μg of doxycycline/ml for 48 h by immunostaining with anti-RINT-1 antibody. −Dox, without doxycycline; +Dox, with doxycycline. Bars, 10 μm. (C) The depletion of RINT-1 inhibited cell growth of the HeLa-RINT-1i clone. Cells were induced with or without 5 μg of doxycycline/ml, and the viable cells were counted by the trypan blue exclusion assay at the indicated times. Each data point represents the average of results for duplicate samples. The data are representative of results from three independent experiments. Error bars, standard deviations. (D) The depletion of RINT-1 caused cell death in a randomly growing population. HeLa-RINT-1i cells were treated with or without 5 μg of doxycycline/ml for the indicated times, and the cell cycle distribution was analyzed by flow cytometry. A significant increase in sub-G1 cells among RINT-1-depleted cells was observed. The data are representative of results from three independent experiments. (E) RINT-1-depleted cells underwent cell death at the G2/M phase. (Top) Diagram showing the experimental scheme for cell cycle synchronization by double thymidine blocking at the G1/S boundary. (Middle) Profiles from fluorescence-activated cell sorter analysis. Cells were collected and analyzed by flow cytometry. 2N, diploid cells; 4N, tetraploid cells. (Bottom) The percentages of cells at each cell cycle phase are indicated. An increase in the sub-G1 population and a decrease in the G2/M population among RINT-1-depleted cells were simultaneously observed. The data are representative of results from three independent experiments. (F) Proliferation curve of one cell cycle division. HeLa-RINT-1i cells were synchronized at mitotic phase by treatment with 0.1 μg of nocodazole/ml for 8 h. The mitotic cells were collected and replated for 4 h, followed by treatment with or without 5 μg of doxycycline/ml. The viable cells were counted by the trypan blue exclusion assay at the indicated times. Each data point represents the average of results for duplicate samples. The data are representative of results from two independent experiments. Error bars, standard deviations. (G) Living HeLa-RINT-1i cells harboring histone H2B-GFP were monitored under a fluorescence microscope at the indicated times after induction with or without 5 μg of doxycycline/ml for 12 h. Chromosome behavior during M phase was recorded. The mitotic progression of control cells is shown in panel a. The mitotic progression of RINT-1-depleted cells is shown in panels b to h. The numbers indicate the minutes passed during the recording. Bars, 10 μm. (H) The mitotic phase of HeLa-RINT-1i cells expressing histone H2B-GFP and treated with doxycycline was prolonged compared to that of control cells. The M phase length was determined from the intervals between chromosome condensation and the completion of cytokinesis. (I) The percentage of HeLa-RINT-1i/H2B-GFP cells treated with doxycycline that died in the mitotic phase was increased compared to that of control cells. Error bars, standard deviations.