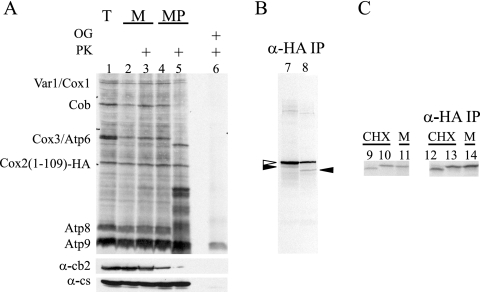
FIG. 3.
Cox2(1-109)-HA remains N-terminally unprocessed following translation in isolated mitochondria. (A) Mitochondrial translation products were radiolabeled with [35S]methionine in mitochondria purified from strain HLF3-60 (lane 1). Labeled mitochondria (lanes 2 and 3) or mitoplasts created through osmotic shock treatment (lanes 4 and 5) were mock treated (lanes 2 and 4) or treated with protease (lanes 3 and 5) (see Materials and Methods). The samples were separated by a 14 to 20% gradient SDS-PAGE, visualized by autoradiography, and probed with antibodies raised against cytochrome b2 (IMS localized) and citrate synthase (matrix localized) as controls for mitoplasting efficiency. A fraction of the total sample was treated with detergent (1% octylglucoside [OG]) and protease (lane 6), as indicated. (B) The samples in lanes 7 and 8 were obtained from the fractions analyzed in lanes 4 and 5, respectively, by immunoprecipitation with anti-HA coupled to beads. The major species corresponded to N-terminally unprocessed peptide (unfilled arrow). A smaller amount of processed Cox2(1-109)-HA (filled arrows) was shortened during protease treatment. (C) Radiolabeled Cox2(1-109)-HA from IMP1 strain HLF3-60 following translation in cycloheximide-treated cells (lane 9) and in isolated organelles (lane 11) was compared to that synthesized in cycloheximide-treated cells from imp1Δ strain HLF4-73 (lane 10) following separation by 14 to 20% gradient SDS-PAGE. The products analyzed in lanes 9 to 11 were immunoprecipitated with anti-HA antibody coupled to beads (in lanes 12 to 14, respectively) to verify the identity of Cox2(1-109)-HA species. While the N terminus of Cox2(1-109)-HA was processed by Imp1 in cycloheximide-treated cells, it remained unprocessed in the imp1Δ mutant and during translation in isolated mitochondria.