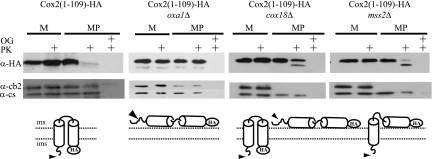
FIG. 4.
The C-terminal domain of Cox2 is not required for integration of the transmembrane domains nor translocation of an HA epitope. Mitochondria (M) carrying full-length Cox2-HA (50 μg) or Cox2(1-109)-HA (200 μg) from strains with wild-type, oxa1Δ, cox18Δ, or mss2Δ nuclear backgrounds was purified from strains HLF3-60, HLF4-17, HLF4-30, and HLF4-63, respectively, and converted to mitoplasts (MP) in the absence or presence of proteinase K (PK). The mitochondrial proteins were resolved by 12% SDS-PAGE, immunoblotted, and decorated with anti-HA antibodies. The size difference between Imp1-processed and unprocessed Cox2(1-109)-HA was not resolved under these electrophoretic conditions. To control for mitoplasting effectiveness, similar blots (containing 5 μg mitochondria) were probed with antibodies against cytochrome b2 and citrate synthase. Solubilization of mitochondrial membranes in 1% octyl glucoside (OG) demonstrated effective protease activity.