Figure 3.
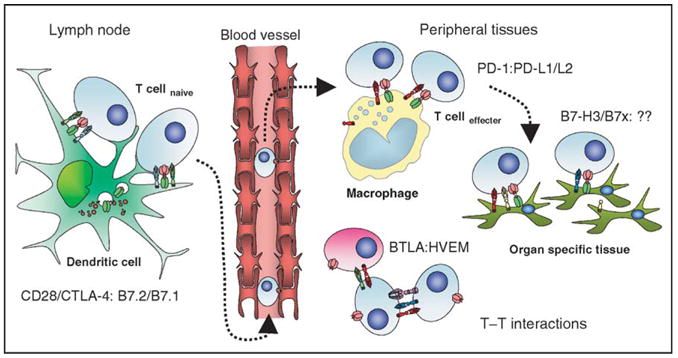
Immunological inhibitory checkpoints: potential therapeutic targets. All of the members of the immunoglobulin superfamily that act as inhibitory checkpoints are potential targets for manipulation in immunotherapies. CD28/CTLA-4:B7.1/B7.2 are centrally important for the initial activation of naïve T cells and regulation of the clonal composition of the responding repertoire following migration of activated dendritic cells to lymphoid organs. As activated effectors traffic back into peripheral tissues they come under the influence of PD1:PD-L1/L2 mediated signaling, both as a result of interactions with tissue macrophages and with ligands expressed on malignant cells. B7-H3 and B7x could be poised to act as the final arbiters of the fate of T effector interactions with nonlymphoid target tissues, and could potentially protect any tumor cells expressing them from cytotoxic T cell mediated killing. The potential for cross-talk between T-cell populations via many of these pathways is complex, particularly as activated T cells can upregulate receptors and/or ligands, which can potentially signal bidirectionally. Blockade of BTLA may remove inhibitory restraints imposed by HVEM-expressing cells, but effects on T–T interactions mediated by blockade of CTLA-4, PD-1/PD-L1, or B7-H3 are also possible.
