Figure 4.
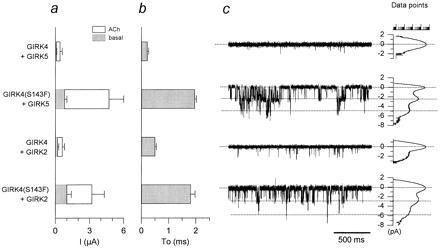
The phenylalanine residue in GIRK4(S143F) alters gating kinetics and enhances activity of heteromers with other GIRK channels. (a) Ba2+-sensitive current magnitude comparisons are shown among coinjections of GIRK5 or GIRK2 with GIRK4(S143F) or GIRK4: GIRK4/GIRK5 (N = 3); GIRK4(S143F)/GIRK5 (N = 3); GIRK4/GIRK2 (N = 4); GIRK4(S143F)/GIRK2 (N = 4). (b) “To” comparison (as in Fig. 3) from activity resulting from coinjection of GIRK5 or GIRK2 with GIRK4 or GIRK4(S143F): GIRK4/GIRK5 (n = 3 patches); GIRK4(S143F)/GIRK5 (n = 10); GIRK4/GIRK2 (n = 3); GIRK4(S143F)/GIRK2 (n = 10). (c) Single-channel currents from representative cell-attached patches from Xenopus oocytes coexpressing GIRK4/GIRK5, GIRK4(S143F)/GIRK5, GIRK4/GIRK2, GIRK4(S143F)/GIRK2, and associated all-point histogram plots are shown. The membrane was held at −80 mV. hm2 receptor (1.5 ng/oocyte) was coinjected with 2 ng/oocyte each of the channel cRNAs tested.
