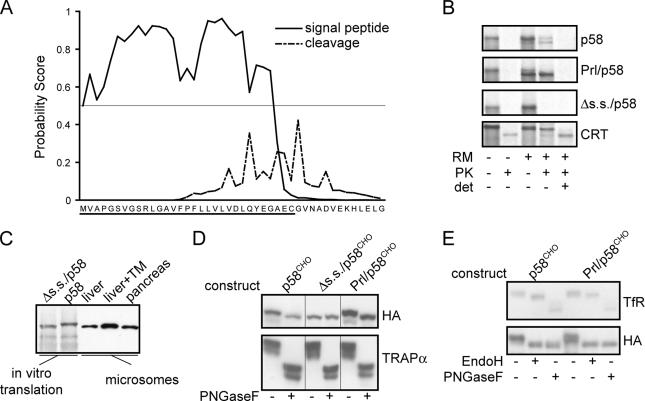
Figure 2.
p58IPK contains a functional N-terminal signal sequence. (A) SignalP3.0 analysis of the murine p58IPK N terminus predicts a likely ER targeting signal (solid line) and cleavage site (broken line). In this analysis, the probability of each residue being part of a targeting signal (based on an artificial neural network trained on known eukaryotic signal sequences) or suitable site for signal peptidase cleavage is plotted (Bendtsen et al., 2004). A horizontal line at the 0.5 probability value for the targeting signal plot is indicated, showing that nearly the entire putative signal sequence of p58IPK (underlined) is above this cut-off. (B) Transcripts encoding the indicated constructs were translated in vitro in the presence or absence of ER-derived rough microsomes (RM). After synthesis, samples were divided into equal aliquots and treated as indicated with 0.5 mg/ml PK in the presence or absence of 1% Triton-X100 (det) to assess translocation into the lumen of microsomes. Note that calreticulin (Crt) contains a core domain that is resistant to digestion by PK. (C) In vitro translations of full-length murine p58 and Δs.s./p58 were analyzed adjacent to microsomes isolated from mouse liver, TM-treated murine liver, and dog pancreas. Detection of p58 was performed by immunoblotting. (D) NIH3T3 cells were transfected with constructs encoding p58CHO, Δs.s./p58CHO, or Prl/p58CHO, each of which is appended at its C-terminus with an HA epitope tag and contains an inserted potential N-linked glycosylation site near the C terminus. Cell lysates were incubated in the absence or presence of PNGaseF before immunoblot with antibodies against the HA epitope or the ER resident glycoprotein TRAPα. (E) Lysates of NIH 3T3 cells transfected as indicated were treated with PNGaseF or EndoH followed by immunoblot against the HA epitope or the cell surface TfR. Resistance to EndoH digestion is indicative of glycan modifications that accompany trafficking of proteins past the cis-Golgi.