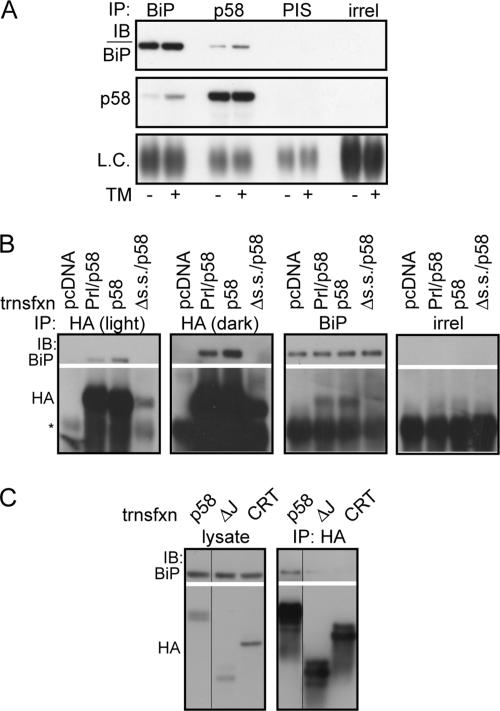
Figure 4.
p58IPK interacts with BiP in the ER lumen. (A) Liver microsomes from nontreated or TM-injected mice were solubilized in 1% Triton X-100 and immunoprecipitated with antiserum against BiP, an antiserum directed against the C terminus of p58IPK, preimmune serum (PIS), or an irrelevant antibody against interferon-γ (irrel). Immune complexes were then probed by immunoblot (IB) for BiP, p58IPK, or immunoglobulin (Ig)G light chain. (B) NIH 3T3 cells were transfected with empty vector, Prl/p58, p58, or Δs.s./p58. Cells were incubated in situ with the membrane-permeable cross-linker DSP at 2 mM, followed by lysis under denaturing conditions and immunoprecipitation with antibodies against BiP, the HA epitope, or an irrelevant antigen (interferon-γ). After cross-linker reversal, the immunoprecipitations were probed by immunoblot as indicated. Asterisk represents IgG heavy chain. Expression of Δs.s./p58 is much lower, likely due to degradation. Thus, a darker exposure is also shown to confirm the absence of an association between this protein and BiP. (C) NIH 3T3 cells were transfected with full-length p58CHO or the same construct with the J-domain deleted (ΔJ). Overexpression of HA-tagged Crt served as a negative specificity control for BiP interaction. Cell lysates were prepared under nondenaturing conditions (no cross-linker was used) and immunoprecipitated with an HA antibody, followed by immunoblot as indicated. Multiple bands for p58CHO constructs represent heterogeneity of glycosylation for the protein.