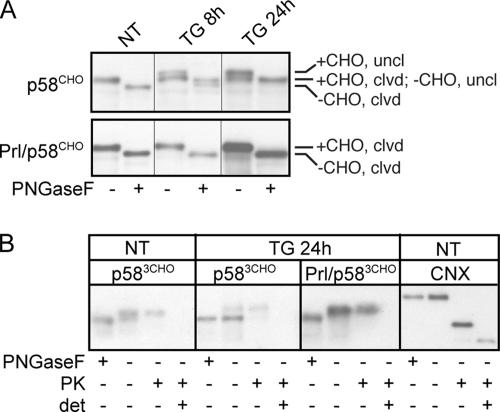
Figure 7.
ER stress reduces the efficiency of p58IPK translocation. (A) NIH 3T3 cells were transfected with p58CHO or Prl/p58CHO and treated with 100 nM TG for the indicated times. Lysates were digested with PNGaseF to resolve N-glycosylated from unmodified chains. Although ER stress does not affect either signal sequence cleavage or glycosylation of Prl/p58CHO, TG inhibits signal sequence cleavage of p58CHO, such that by 24 h all chains exist in the uncleaved (uncl) state. Of the uncleaved chains, approximately half are glycosylated (+CHO) and half unglycosylated (−CHO). (B) NIH 3T3 cells were transfected with p583CHO or Prl/p583CHO (identical to the constructs used in A except containing three C-terminal N-linked glycosylation sites to better separate glycosylated from nonglycosylated material) and treated with 100 nM TG for 24 h as indicated. Hypotonic lysates were prepared and treated with PK as described in Figure 3A, or PNGaseF as described above. p583CHO and Prl/p583CHO were identified by immunoblotting. Note that ER stress causes a substantial portion of p583CHO chains, but not Prl/p583CHO, to be unglycosylated, and these unglycosylated chains are sensitive to PK digestion, whereas glycosylated chains are resistant. Immunoblotting to detect a lumenal epitope of endogenous calnexin (CNX) confirmed the proper orientation and integrity of the membranous vesicles within the homogenate.