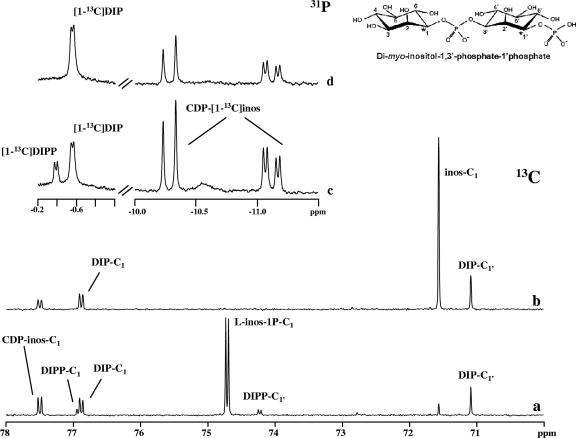
FIG. 3.
13C-NMR and 31P-NMR spectra showing the formation of 13C-labeled products in a cell extract of A. fulgidus after incubation at 80°C. Traces a and c represent spectra of the reaction mixture to which CTP and l-[1-13C]myo-inositol-1-phosphate were added; traces b and d represent spectra of the same sample after treatment with alkaline phosphatase. A schematic representation of DIPP is also shown. Spectra were run at a temperature of 25°C. inos-C1, l-inos-1P-C1, DIP-C1, DIPP-C1, and CDP-inos-C1 designate resonances due to carbon 1 of the inositol moiety in myo-inositol, l-myo-inositol-1-P, DIP, DIPP, and CDP-l-myo-inositol. The splitting of the carbon (or phosphorus) resonances denotes two-bond couplings with phosphorus (or carbon) nuclei.