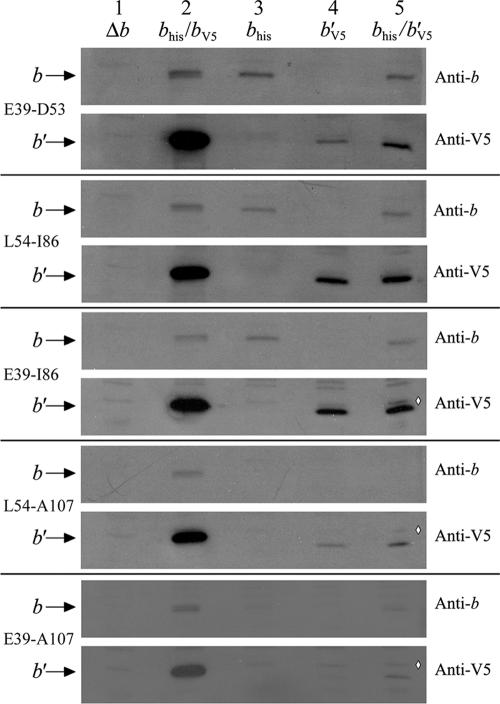
FIG. 2.
Immunoblot of membranes prepared from E. coli strain KM2 (Δb) expressing chimeric b subunits. Aliquots of membrane proteins (1 μg) were separated on 15% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes as previously described (14). The presence of the b subunit was detected using both a polyclonal antibody raised against the b subunit and an antibody against the V5 epitope tag appended to the carboxyl terminus of the chimeric b′ subunits. The position of the b subunit band and the region that was replaced with T. elongatus sequence are indicated on the left and the primary antibody on the right. Membrane samples were loaded as follows: lane 1, negative control KM2/pBR322 (Δb); lane 2, positive control KM2/pTAM37/pTAM46 (bhis/bV5); lane 3, chimeric bhis subunits; lane 4, chimeric b′V5 subunits; lane 5, coexpression of chimeric bhis and b′V5 subunits. The commercial anti-V5 antibody gave a much stronger signal than the polyclonal anti-b subunit antibody and also detected an extra band running near the level of the b subunit, indicated in lane 5 by a white diamond (⋄).