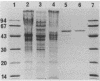
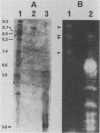
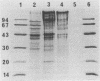
Abstract
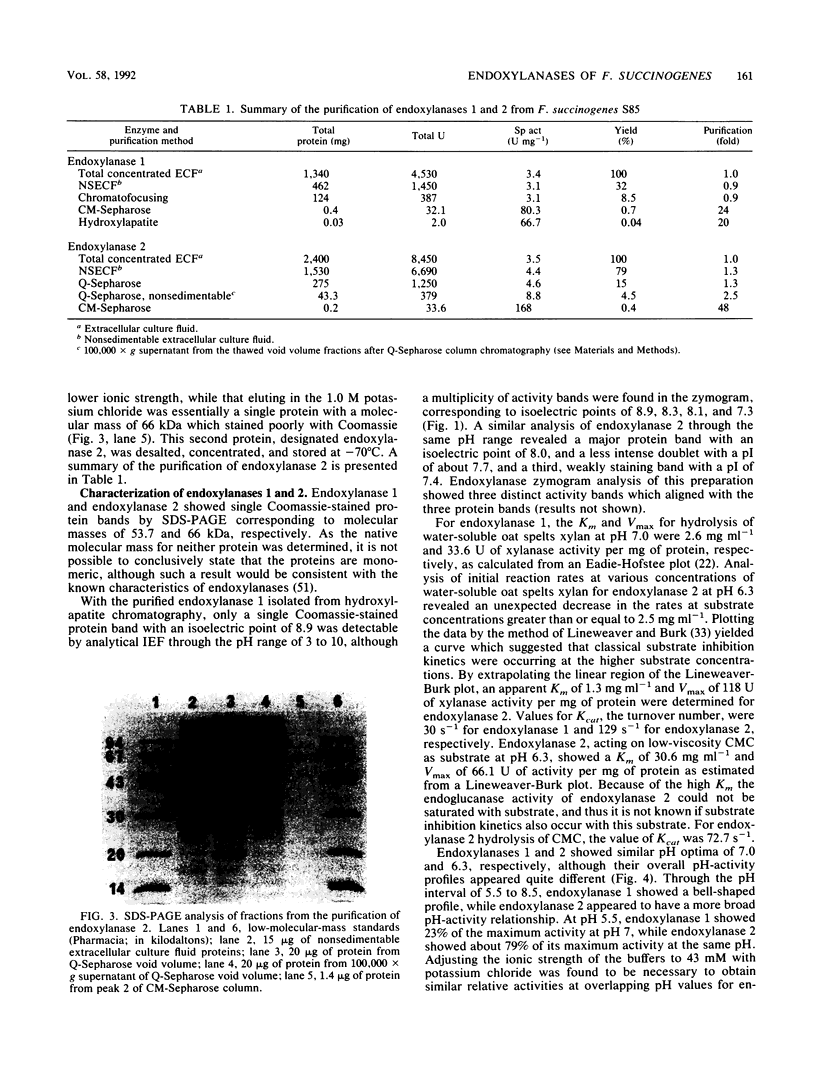
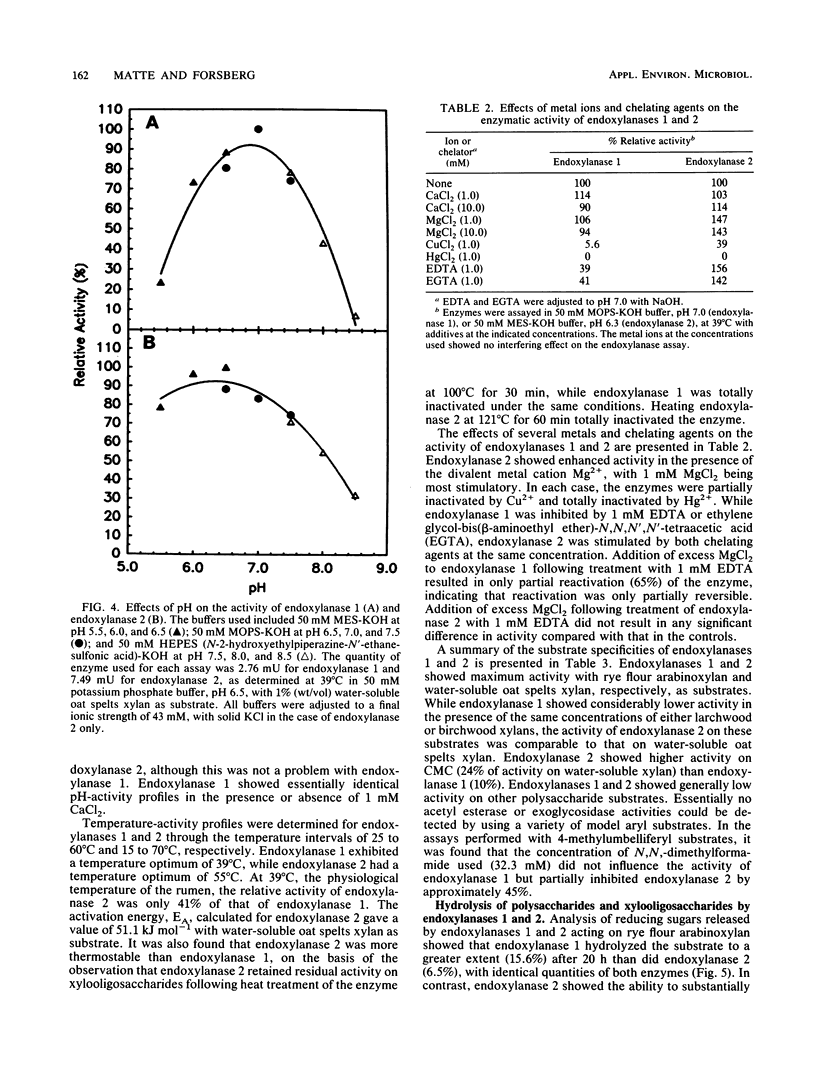
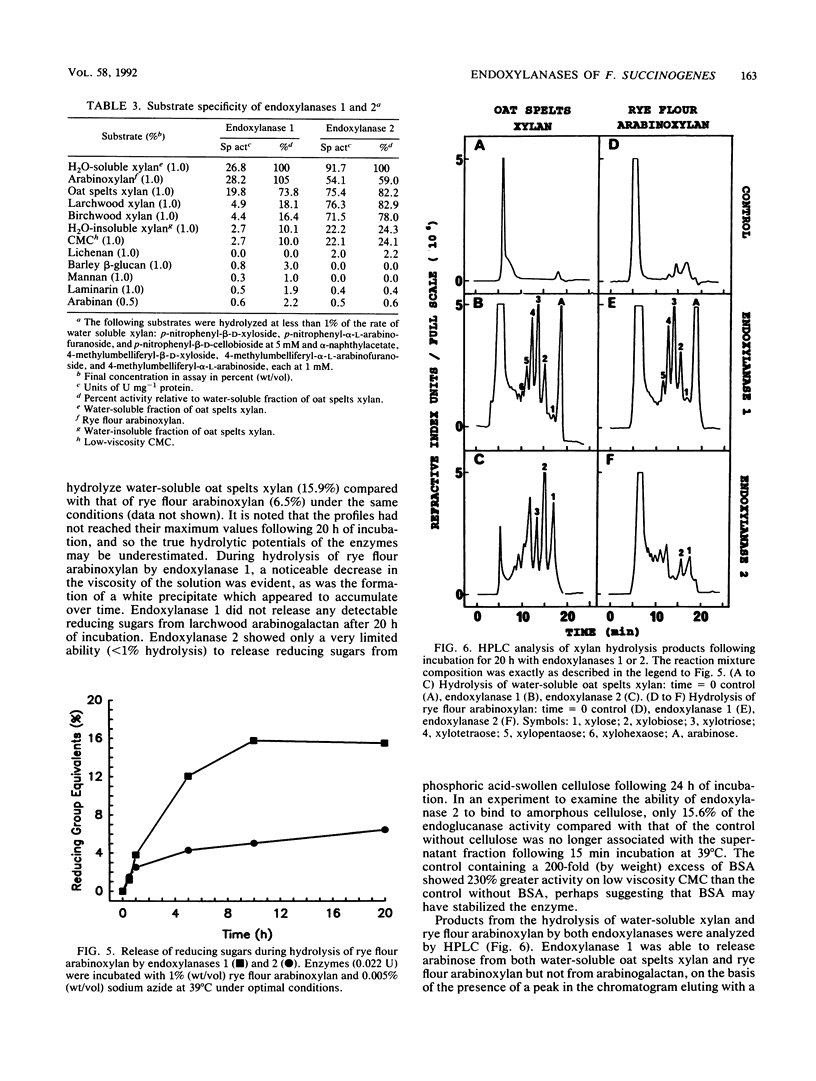
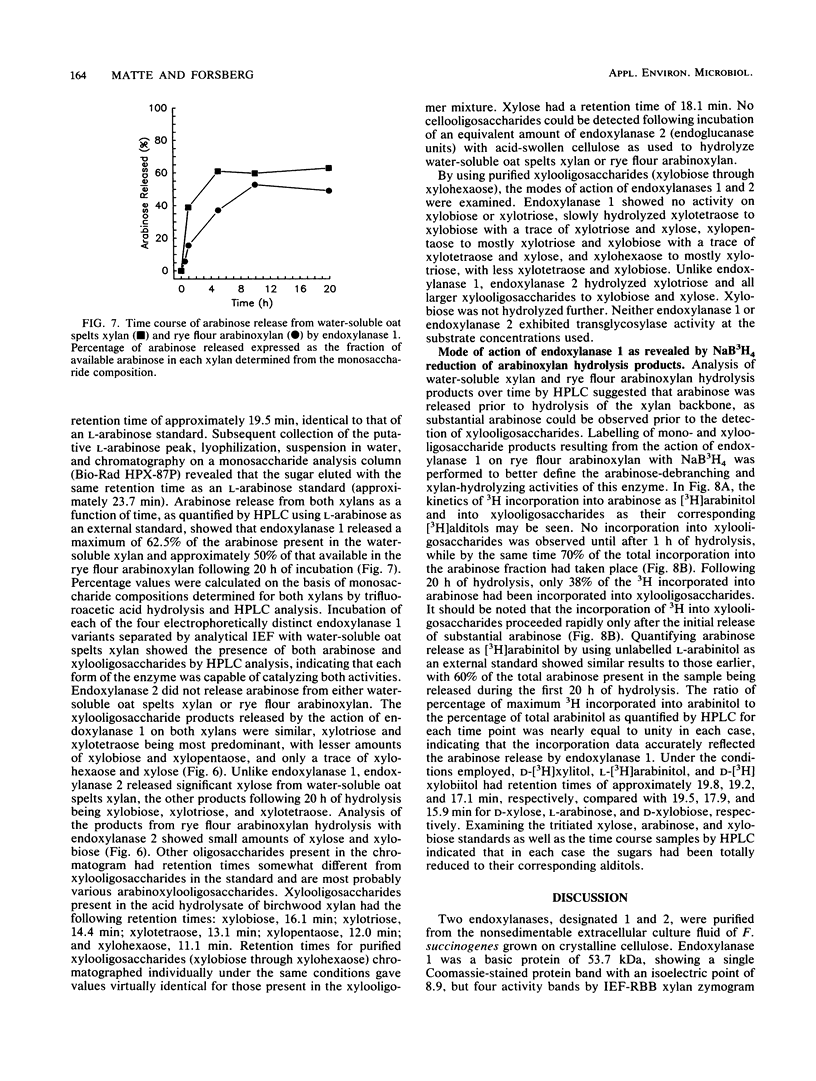
Two different endoxylanases (1,4-beta-D-xylan xylanohydrolases, EC 3.2.1.8), designated 1 and 2, have been purified by column chromatography to apparent homogeneity from the nonsedimentable extracellular culture fluid of the strictly anaerobic, ruminal bacterium Fibrobacter succinogenes S85 grown on crystalline cellulose. Endoxylanases 1 and 2 were shown to be basic proteins of 53.7 and 66.0 kDa, respectively, with different pH and temperature optima, as well as different substrate hydrolysis characteristics. The Km and Vmax values with water-soluble oat spelts xylan as substrate were 2.6 mg ml-1 and 33.6 mumol min-1 mg-1 for endoxylanase 1 and 1.3 mg ml-1 and 118 mumol min-1 mg-1 for endoxylanase 2. Endoxylanase 1, but not endoxylanase 2, released arabinose from water-soluble oat spelts xylan and rye flour arabinoxylan, but not from arabinan, arabinogalactan, or aryl-alpha-L-arabinofuranosides. With an extended hydrolysis time, endoxylanase 1 released 62.5 and 50% of the available arabinose from water-soluble oat spelts xylan and rye flour arabinoxylan, respectively. Endoxylanase 1 released arabinose directly from the xylan backbone, and this preceded hydrolysis of the xylan to xylooligosaccharides. Endoxylanase 2 showed significant activity against carboxymethyl cellulose but was unable to substantially hydrolyze acid-swollen cellulose. Both enzymes were endo-acting, as revealed by their hydrolysis product profiles on water-soluble xylan and xylooligosaccharides. Because of their unique hydrolytic properties, endoxylanases 1 and 2 appear to have strategic roles in plant cell wall digestion by F. succinogenes in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniou T., Marquardt R. R., Cansfield P. E. Isolation, partial characterization, and antinutritional activity of a factor (pentosans) in rye grain. J Agric Food Chem. 1981 Nov-Dec;29(6):1240–1247. doi: 10.1021/jf00108a035. [DOI] [PubMed] [Google Scholar]
- Biely P., Markovic O., Mislovicová D. Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels. Anal Biochem. 1985 Jan;144(1):147–151. doi: 10.1016/0003-2697(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Biely P., Mislovicová D., Toman R. Soluble chromogenic substrates for the assay of endo-1,4-beta-xylanases and endo-1,4-beta-glucanases. Anal Biochem. 1985 Jan;144(1):142–146. doi: 10.1016/0003-2697(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Forsberg C. W., Beveridge T. J., Hellstrom A. Cellulase and Xylanase Release from Bacteroides succinogenes and Its Importance in the Rumen Environment. Appl Environ Microbiol. 1981 Nov;42(5):886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski A. C., Jeffries T. W. Production, Purification, and Characterization of beta-(1-4)-Endoxylanase of Streptomyces roseiscleroticus. Appl Environ Microbiol. 1991 Apr;57(4):987–992. doi: 10.1128/aem.57.4.987-992.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Hungate R. E. alpha-L-arabinofuranosidase from Ruminococcus albus 8: purification and possible role in hydrolysis of alfalfa cell wall. Appl Environ Microbiol. 1984 May;47(5):1135–1140. doi: 10.1128/aem.47.5.1135-1140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. Non-inverted versus inverted plots in enzyme kinetics. Nature. 1959 Oct 24;184:1296–1298. doi: 10.1038/1841296b0. [DOI] [PubMed] [Google Scholar]
- HOWARD B. H., JONES G., PURDOM M. R. The pentosanases of some rumen bacteria. Biochem J. 1960 Jan;74:173–180. doi: 10.1042/bj0740173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., O'Bryan-Shah P. J. Esterase activities in Butyrivibrio fibrisolvens strains. Appl Environ Microbiol. 1988 Aug;54(8):1917–1922. doi: 10.1128/aem.54.8.1917-1922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Purification and Comparison of the Periplasmic and Extracellular Forms of the Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1988 Jun;54(6):1488–1493. doi: 10.1128/aem.54.6.1488-1493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W., Thomas D. Y. Purification and characterization of a chloride-stimulated cellobiosidase from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2923–2932. doi: 10.1128/jb.170.7.2923-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluepfel D., Vats-Mehta S., Aumont F., Shareck F., Morosoli R. Purification and characterization of a new xylanase (xylanase B) produced by Streptomyces lividans 66. Biochem J. 1990 Apr 1;267(1):45–50. doi: 10.1042/bj2670045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Gibbins L. N. Xylanolytic Activity of Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Oct;50(4):1068–1076. doi: 10.1128/aem.50.4.1068-1076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Forsberg C. W., Rattray J. B. Purification and Characterization of Two Endoxylanases from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1987 Apr;53(4):644–650. doi: 10.1128/aem.53.4.644-650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie C. R., Yang R. C., Patel G. B., Bilous D., Narang S. A. Identification of three distinct Clostridium thermocellum xylanase genes by molecular cloning. Arch Microbiol. 1989;152(4):377–381. doi: 10.1007/BF00425176. [DOI] [PubMed] [Google Scholar]
- McDermid K. P., Forsberg C. W., MacKenzie C. R. Purification and properties of an acetylxylan esterase from Fibrobacter succinogenes S85. Appl Environ Microbiol. 1990 Dec;56(12):3805–3810. doi: 10.1128/aem.56.12.3805-3810.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermid K. P., Mackenzie C. R., Forsberg C. W. Esterase Activities of Fibrobacter succinogenes subsp. succinogenes S85. Appl Environ Microbiol. 1990 Jan;56(1):127–132. doi: 10.1128/aem.56.1.127-132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M. J., Forsberg C. W., Crosby B., Bell A. W., Dignard D., Thomas D. Y. Structure of the cel-3 gene from Fibrobacter succinogenes S85 and characteristics of the encoded gene product, endoglucanase 3. J Bacteriol. 1989 Oct;171(10):5587–5595. doi: 10.1128/jb.171.10.5587-5595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P. J. Xylanases: structure and function. Basic Life Sci. 1981;18:111–129. doi: 10.1007/978-1-4684-3980-9_8. [DOI] [PubMed] [Google Scholar]
- SCOTT H. W., DEHORITY B. A. VITAMIN REQUIREMENTS OF SEVERAL CELLULOLYTIC RUMEN BACTERIA. J Bacteriol. 1965 May;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipat A., Taylor K. A., Lo R. Y., Forsberg C. W., Krell P. J. Molecular cloning of a xylanase gene from Bacteroides succinogenes and its expression in Escherichia coli. Appl Environ Microbiol. 1987 Mar;53(3):477–481. doi: 10.1128/aem.53.3.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenath H. K., Joseph R. Purification and properties of extracellular xylan hydrolases of Streptomyces exfoliatus. Folia Microbiol (Praha) 1982;27(2):107–115. doi: 10.1007/BF02879768. [DOI] [PubMed] [Google Scholar]
- Wong K. K., Tan L. U., Saddler J. N. Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev. 1988 Sep;52(3):305–317. doi: 10.1128/mr.52.3.305-317.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]