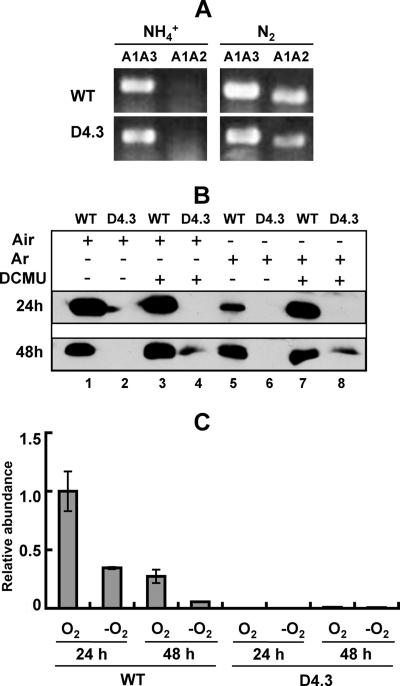
FIG. 3.
Expression of nifH in the wild type and D4.3. (A) Rearrangement of the nifD element in the wild type (WT) and the strain D4.3. Primers A1 and A3 (A1A3) were used to amplify the DNA fragment corresponding to the nonrearranged DNA, and primers A1 and A2 (A1A2) were used to amplify the DNA fragment corresponding to the rearranged DNA. Cells were cultured for 3 days in the presence of ammonium (NH4+) and then incubated for 18 h in the absence of combined nitrogen (N2). DNA was extracted and used as template for PCR amplification. PCR products were loaded on an 0.8% agarose gel and separated by electrophoresis. (B) Immunodetection of NifH in the wild type (WT) and D4.3. Filaments were cultured in the absence of fixed nitrogen for 24 or 48 h and then incubated under four conditions: lanes 1 and 2, in the air; lanes 3 and 4, in the air and the presence of DCMU; lanes 5 and 6, in argon (Ar) instead of air; lanes 7 and 8, in argon instead of air and in the presence of DCMU. Fifty micrograms of total protein was separated on a 15% sodium dodecyl sulfate-polyacrylamide gel, transferred onto a nitrocellulose membrane, and incubated with antibodies against NifH. (C) The relative abundance of nifH transcripts determined by real-time quantitative PCR. The amounts of nifH transcripts were normalized to those of rnpB as an internal standard. The amount of the nifH transcripts measured under oxic conditions with wild-type cells at 24 h after the deprivation of combined nitrogen was set at 1, and all the other data were calculated relative to this value.