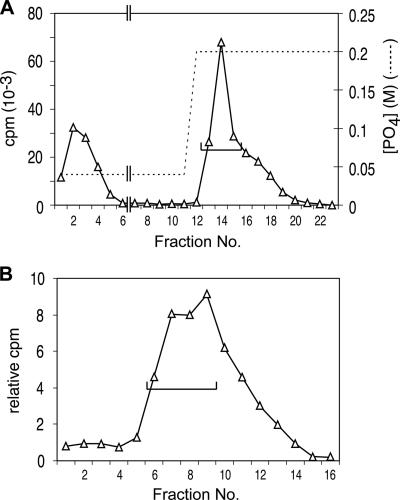
FIG. 1.
Initial fractionation of cell extract. (A) An 800-ml culture of R1501 (3) in C medium supplemented with bovine serum albumin and CaCl2 at pH 7.9 (9) was treated with 80 μg of CSP-1 at an optical density of 0.11 at 37°C for 12 min. The competent culture was cooled to 25°C by agitation in ice-water for 64 s in two 500-ml Schott bottles, exposed to 160 μg [3H]DNA (20,000 cpm μg−1; uniformly labeled chromosomal DNA prepared as described previously [5]) for 6 min and then to 3 mg DNase I for 30 s, and cooled further for centrifugation at 5,000 × g for 15 min. The pellet was washed with 40 ml cold SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and resuspended in 8 ml lysis buffer containing 0.1 M NaCl, 0.05 M Tris-HCl (pH 7.5), 0.01 M EDTA, 0.5% Sarkosyl, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 50 μg ml−1 RNase A, and 10% glycerol. The clear lysate obtained after 5 min at 37°C was passed 20 times through a 5-cm 21G hypodermic needle to reduce viscosity. The sheared lysate was brought to 0.04 M PB (pH 6.8) and loaded on a 9-ml HAP column. The column was washed at room temperature with 3.3 column volumes of 0.05% Sarkosyl-0.04 M PB, followed by 23 ml of the same buffer adjusted to 0.20 M PB. The flowthrough and subsequent eluates were collected in 4-ml (lanes 1 to 8) or 2-ml (lanes 9 to 23) fractions. Total numbers of cpm per fraction are displayed. Peak fractions (lanes 13 to 15) containing ∼40% of donor DNA label (bracket) were pooled. (B) S400 gel exclusion fractionation. Pooled fractions from panel A (6 ml) were applied to a 48-ml (1.5-cm diameter) column of Sephacryl S400HR and eluted with 0.15 M NaCl-0.05% Sarkosyl at 0.25 ml min−1. The first 16 2-ml fractions collected are shown. Fractions 6 to 9 were combined for analysis by gradient elution from HAP.