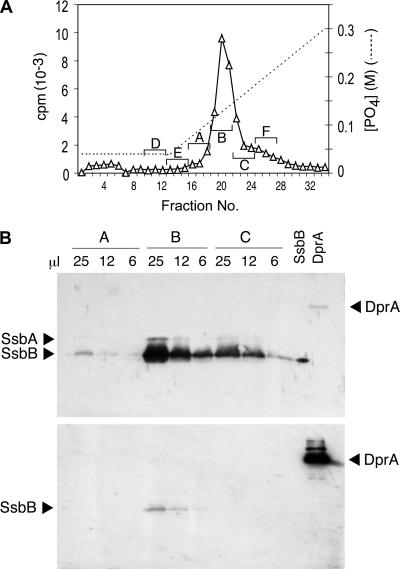
FIG. 2.
Identification of the major EC protein. (A) HAP chromatography of EC. The S400HR excluded-volume peak was brought to 0.04 M PB and loaded on a 2-ml HAP column; the column was washed with 9 ml 0.04 M PB and eluted with a 40-ml linear gradient of PB from 0.04 to 0.3 M PB. The nominal PB gradient was estimated for each collected fraction. Three-fraction pools were made as indicated by letters and brackets and concentrated 40-fold by centrifugal ultrafiltration (Amicon Centricon; 10,000-Da MM cutoff) with 0.05% SDS and 100 ng bovine serum albumin. (B) Western blot analyses were classically performed as described in reference 11, using samples of the indicated volumes from 100-μl pools A, B, and C (from panel A); 10 ng of purified SsbB protein and 20 ng of purified DprA protein (20) were included as controls. Rabbit polyclonal antibodies raised against purified Bacillus subtilis SSB were used at a 1/2,000 serum dilution to detect SsbB and SsbA proteins (upper). Antibodies were then stripped off, and the membrane was incubated with rabbit polyclonal anti-DprA antibodies raised against two synthetic peptides of DprA (residues 3 to 18 and residues 118 to 131) at a 1/5,000 serum dilution to detect DprA protein (lower) (note that the band observed at the position of SsbB might correspond to incomplete removal of anti-SSB antibodies).