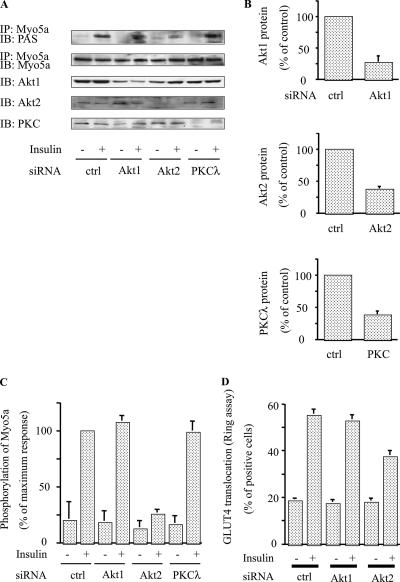
FIG. 5.
Akt2, but not Akt1 or PKCλ, knockdown abolished phosphorylation of myosin 5a. (A to C) Knockdown of Akt2 decreased insulin-stimulated phosphorylation of myosin 5a. 3T3-L1 adipocytes were electroporated with the indicated siRNA, starved, and incubated in the absence (-) or presence (+) of 17 nM insulin. Then, the cells were lysed and Western blotting was performed with anti-Akt1, anti-Akt2, and anti-PKCλ antibodies. The same lysates were subjected to immunoprecipitation (IP) with anti-myosin 5a antibody. The washed immunoprecipitation products were analyzed by Western blotting with anti-PAS antibody. The membrane was stripped and reblotted with anti-myosin 5a antibody (A). (B) Data are presented as percentages of protein content compared to control siRNA-electroporated cells and represent the mean ± SEM from three independent experiments. (C) Data are presented as percentages of phosphorylation compared to insulin-stimulated control siRNA-electroporated cells and represent the mean ± SEM from three independent experiments. (D) Knockdown of Akt2 decreased insulin-stimulated GLUT4 translocation. The microinjected cells were stimulated with (+) or without (-) 17 nM insulin. Cell surface GLUT4 was determined by staining as described in Materials and Methods. Data shown are the mean ± SEM of results from three independent experiments. IB; immunoblot.