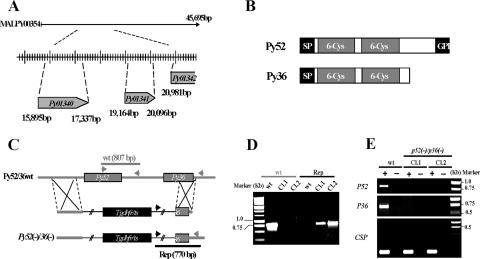
FIG. 1.
Targeted gene disruption of P52 and P36 using a single-replacement strategy. (A) P. yoelii P52 (PY01340) and P36 (PY01341) are located in tandem on contig MALPY00354. (B) Predicted protein structure of P52 and P36. Each protein exhibits a signal peptide (SP) followed by two 6-Cys domains. In addition, P52 possesses a putative GPI anchor transfer peptide. (C) Schematic representation of the replacement strategy to generate the p52/p36-deficient parasites. The wild-type (wt) P52/P36 genomic locus was targeted with a replacement plasmid containing a 5′ untranslated region fragment of P52 and a 3′ fragment of the P36 ORF that flank the Toxoplasma gondii dihydrofolate reductase-thymidylate synthase-positive selectable marker. A recombination event (double crossover) resulted in the replacement of the P52 ORF and the 5′ part of the P36 ORF by the selection marker. wt and replacement-specific oligonucleotide primer combinations used for genotyping are indicated by arrows, and expected fragments are shown by gray and black lines. (D) PCR genotyping. Amplification with oligonucleotide primer combinations that can amplify only from the recombinant locus (Rep) confirmed the gene replacement. The wt-specific oligonucleotide primer combinations confirmed the absence of residual wt parasites in p52/p36-deficient Cl1 and Cl2. (E) The p52/p36-deficient parasites do not transcribe P52 and P36. The absence of P52 and P36 transcripts in p52/p36-deficient parasites was shown by RT-PCR using gene-specific oligonucleotide primers and salivary gland sporozoite RNA as a template. Gene-specific oligonucleotide primers for CSP were used as a positive control and amplified from wt and p52/p36-deficient sporozoite RNA. − indicates reactions without RT, and + indicates reactions with RT.