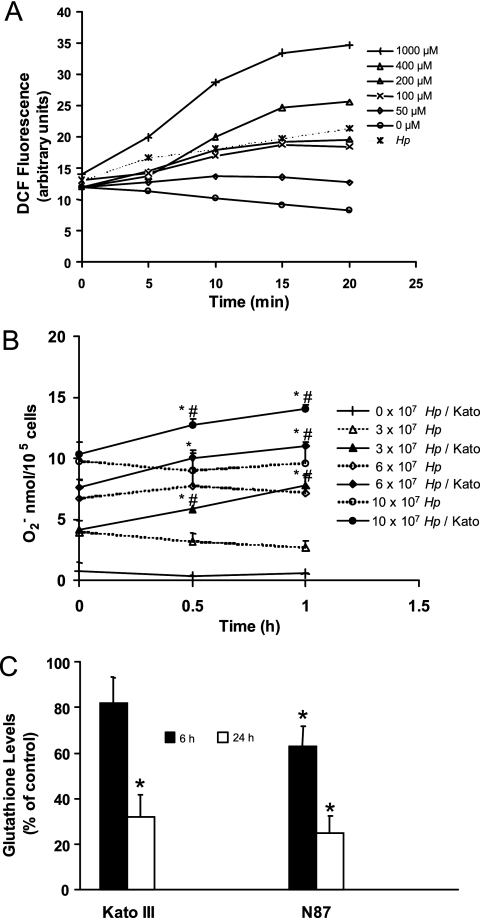
FIG. 1.
Induction of ROS in gastric epithelial cells after exposure to H. pylori or oxygen metabolites. (A) DCFH2-DA-treated Kato III cells were exposed to H2O2 at concentrations from 0 to 1,000 μM or infected with H. pylori (Hp) at a ratio of bacteria to epithelial cells of 300:1. Intracellular DCF fluorescence was measured by flow cytometry at intervals up to 20 min after stimulation. ROS accumulated in proportion to the concentration of H2O2 added to the cells, and bacteria stimulated the production of ROS in epithelial cells in a time-dependent manner. A representative experiment is shown. (B) Superoxide anion was measured by the cytochrome c reduction assay method at 0, 0.5, and 1 h after various concentrations of H. pylori strain 26695 (equivalent to ratios of bacteria to epithelial cells of 0:1, 300:1, 600:1, and 1,000:1) were added to wells containing media alone (dotted line) or media with Kato III gastric epithelial cells (continuous line). A dose-dependent increase in superoxide anion generation was measured with increasing concentrations of bacteria both with and without epithelial cells. Data represent superoxide anion production expressed as means ± SEM (n = 8 to 12). *, P < 0.05 compared to bacteria alone; #, P < 0.05 compared to Kato III cells alone. (C) Kato III and NCI-N87 gastric epithelial cells were exposed to H. pylori (ratio of bacteria to epithelial cells of 300:1), harvested at 6 or 24 h poststimulation, and assayed for levels of intracellular GSH using a colorimetric assay. Oxidative stress generated by H. pylori results in a sustained decrease in GSH levels. The data are means ± SEM, expressed as percentages of control levels. *, P < 0.05 compared to control (n = 5 to 7).