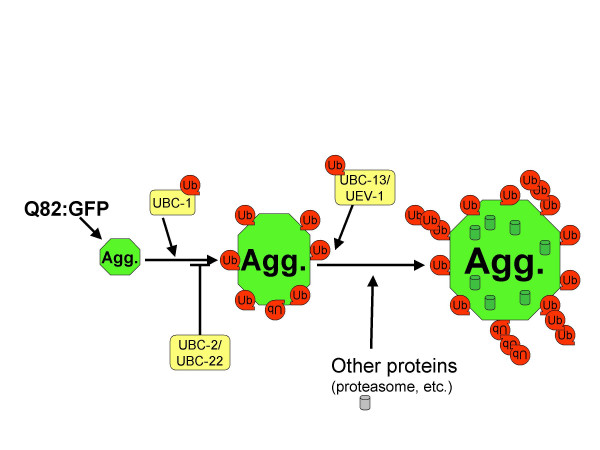
Figure 6.
A model for the role of Ubc's in the formation of polyglutamine aggregates. In our experimental system, Ubc's are required for polyglutamine aggregates to achieve their normal size. Other research has indicated that aggregates initiate as small particles of aggregating protein that then travel along microtubules to ultimately collect and form large aggregates [42]. Since the knockdown of ubc-1 results in smaller, more numerous aggregates, ubiquitination by ubc-1 may be needed for small aggregates to come together and form large aggregates. UBC-2 and UBC-22 may normally have an inhibitory role in this process because their knockdown results in larger and less numerous aggregates. Ubiquitination by ubc-13 and uev-1 may be required to recruit other proteins, such as proteasomes, to the aggregates, thereby allowing aggregates to further increase in size.