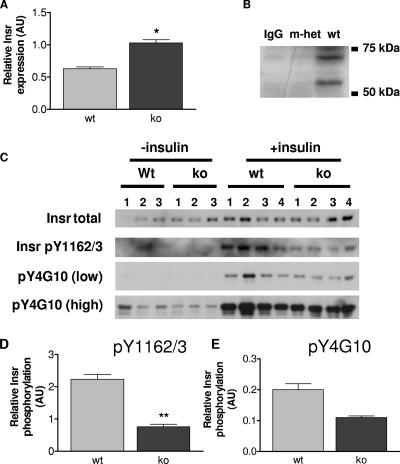
FIG. 7.
Insr levels and tyrosine phosphorylation in quadriceps muscles from wild-type (wt) and Grb10Δ2-4m/p (ko) mice. AU, arbitrary units. (A) Insr levels in the absence of insulin treatment. Tissue lysates were Western blotted with antibodies that recognize all forms of the Insr. Following densitometric analysis, total Insr levels were normalized using total extracellular signal-regulated kinase as a loading control. (B) Coimmunoprecipitation of Grb10 with the Insr. Bands of the expected size for Grb10 (located between markers at 50 kDa and 75 kDa) were detected in skeletal muscle samples from wild-type animals (wt) but not Grb10Δ2-4m/+ animals (m-het) following immunoprecipitation with an anti-Insr antibody and then Western blotting with an anti-Grb10 antibody. Similarly, no Grb10 protein was detected when the anti-Insr antibody was omitted from the immunoprecipitation reaction (immunoglobulin G control [IgG]). (C) Insr tyrosine phosphorylation. Following immunoprecipitation of the Insr, Western blots were probed with antibodies that recognize either Insr phosphorylated within the activation loop (pY1162/3) or all tyrosine-phosphorylated forms of the Insr (pY4G10). Both low and high exposures of the same blot are shown for the pY4G10 antibody. Immunoprecipitates were also Western blotted for total Insr content. (D) Insr phosphorylation within the activation loop, normalized for total Insr levels. (E) Levels of all tyrosine-phosphorylated forms of Insr, normalized for total Insr levels. All results are expressed as means ± SEM (*, P < 0.05; **, P < 0.01 [Student's t test]).