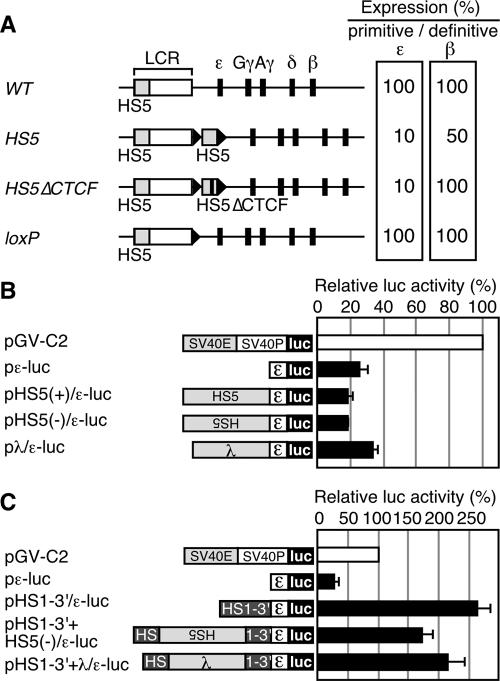
FIG. 1.
(A) Relative expression of the primitive ɛ-globin or the definitive β-globin genes in YAC TgM. The values in the WT TgM are set at 100. Results from the WT (28), HS5 (containing a 2.6 kbp HS5 fragment inserted 3′ to HS1) (30), HS5▵CTCF (containing the HS5 fragment lacking CTCF binding sites), and loxP (same as the WT, except that a ∼40-bp loxP footprint (arrowhead) is retained) TgM are shown. (B) Analysis of potential silencer activity associated with HS5 in K562 cells. Structures of the reporter constructs used in the transfection assays are shown on the left. The human ɛ-globin promoter region (174 to +38) was subcloned into the luciferase reporter plasmid to generate pɛ-luc. The HS5 DNA fragment (2.6 kbp; same as the one used in the HS5 TgM) was inserted upstream of the ɛ-globin promoter in sense or antisense orientations to generate pHS5±/ɛ-luc. A 2.3-kbp fragment from λ DNA was inserted upstream of the ɛ-globin gene promoter to generate pλ/ɛ-luc. The reference reporter construct pGV-C2 is driven by the simian virus 40 (SV40) enhancer (E) and promoter (P). K562 cells were transfected with the luciferase reporter plasmids as well as the control pCMV-β-Gal. Luciferase activities were normalized to β-Gal activities to control for transfection efficiencies. The relative luciferase activity is expressed relative to that in the pGV-C2 (set at 100). Each value of luciferase activity represents the mean ± SD for at least three independent experiments. (C) HS5 DNA insertion does not attenuate the enhancer activity localized within the 3′ region of HS1 (HS1-3′). The HS1-3′ DNA fragment (∼1 kbp) was inserted upstream of the ɛ-globin promoter, within the context of pɛ-luc, to generate pHS1-3′/ɛ-luc. The HS5 (2.6 kbp) or the λ (2.3 kbp) DNA fragment was inserted in the HS1-3′ region (at the same position as the HS5 fragment was inserted in the HS5 TgM) of pHS1-3′/ɛ-luc to generate pHS1-3′+HS5(−)/ɛ-luc and pHS1-3′+λ/ɛ-luc, respectively. These reporter plasmids were transfected into K562 cells and assayed as described above.