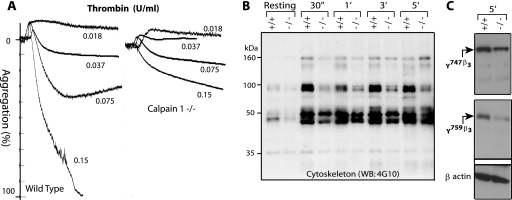
FIG. 1.
Platelet aggregation and tyrosine phosphorylation of cytoskeletal proteins in calpain-1 null platelets. (A) Gel-filtered (3 × 108/ml) platelets were analyzed for platelet aggregation assay in response to different doses of thrombin. (B) Gel-filtered platelets (5 × 108/ml) from WT (+/+) and calpain-1 null (−/−) mice were activated by 0.15 U/ml of thrombin, and tyrosine phosphorylation of the cytoskeletal proteins was analyzed by Western blotting using the 4G10 antibody (WB: 4G10). The cytoskeleton extract was normalized after activation with thrombin for up to 5.0 min (5′) using β-actin as a control antibody. 30″, 30 s. (C) Tyrosine phosphorylation of the β3 subunit of αIIbβ3 integrin was evaluated after 5 min of thrombin activation (0.15 U/ml) of cells from WT (+/+) and calpain-1 null (−/−) mice using antiphosphotyrosine specific antibodies as described in the text. The antibody against β-actin served as a control.