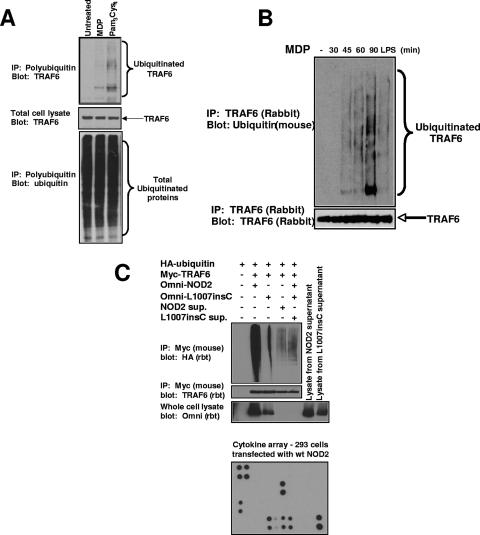
FIG. 4.
MDP causes the activation of endogenous TRAF6. (A) THP-1 cells were stimulated with either MDP (10 μg/ml) or PC (1 μg/ml) or were left untreated. Cells were lysed in RIPA buffer and were immunopurified using antiubiquitin-Sepharose beads (Pierce Biotech.). These antiubiquitin beads bind to polyubiquitin chains containing more than four ubiquitin molecules. The immunoprecipitated polyubiquitin proteins were subjected to SDS-PAGE, and Western blotting was performed using an anti-TRAF6 antibody (upper blot). The total cell lysates was also probed for TRAF6 expression as a control for equivalent starting amounts of TRAF6 (middle blot) and for equivalent amounts of precipitated ubiquitinated proteins (lower blot). (B) MDP stimulates the autoubiquitination of endogenous TRAF6. The mouse macrophage cell line RAW264.7 was left untreated or stimulated with MDP (10 μg/ml) or purified LPS (10 ng/ml) for the time periods indicated. Cells were lysed in RIPA buffer and were immunoprecipitated using anti-TRAF6 antibody (Santa Cruz). The immunoprecipitated polyubiquitinated proteins were subjected to SDS-PAGE, and Western blotting was performed using an antiubiquitin antibody (clone P4D1). The immunoprecipitates were also probed with an anti-TRAF6 antibody to ensure that equivalent amounts of TRAF6 were immunoprecipitated. (C) To determine if NOD2 caused TRAF6 activation indirectly through a paracrine or autocrine loop, NOD2 and the Crohn's disease-associated allele, L1007insC, were transfected into HEK293 cells. One millilter of the medium from these transfectants was subjected to cytokine array analysis (Ray Biotech; bottom panel) showing high levels of IL-8, MCP-1, angiopoietin, and VEGF. The relative positions of the cytokines are shown in Fig. S3 in the supplemental material. Three milliliters of this medium was then added to cells transfected with mouse myc-tagged TRAF6 and rabbit (rbt) HA-tagged ubiquitin. After 30 min, the cells were lysed and TRAF6 was immunoprecipitated. As a positive and negative control, TRAF6 was also cotransfected with wt NOD2 and L1007insC NOD2. Western blotting again showed a strong TRAF6 ubiquitination when wt NOD2 was cotransfected; however, a much weaker TRAF6 ubiquitination was seen when the cells were exposed to medium from cells transfected with wt NOD2. The overexposed blot is shown to highlight the difference. In addition, no change in TRAF6 ubiquitination was identified between cells exposed to media from cells transfected with wt NOD2 or L1007insC NOD2. sup., supernatant.