FIGURE 1. Locations of the mutations in the αVβ 3 crystal structure (3).
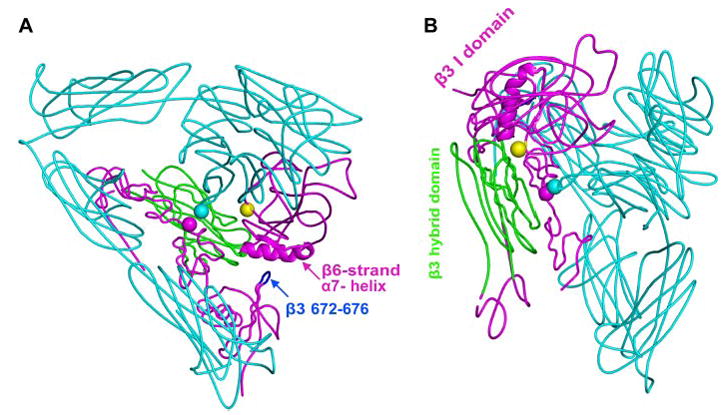
The αV subunit is in cyan. The β3 hybrid domain is in green, and other β3 domains are in magenta. The β3 tail domain CD loop (residues Asp-672–Lys-676) is in blue. Residues mutated to cysteine are shown with spheres at the positions of αV G307 Cα (cyan) and β3 R563 Cα (magenta). The position of the N-glycan wedge introduced by the β3 NIN305T mutation is shown with a yellow sphere at Asn-303 Cα. A, this view emphasizes the β-I domain β6-strand and α7-helix, the only elements shown as a ribbon, and their proximity to the β-tail domain CD loop. B, this view is rotated relative to A and emphasizes the glycan wedge introduced into a crevice between the hybrid and β-I domain that widens in the open headpiece conformation.
