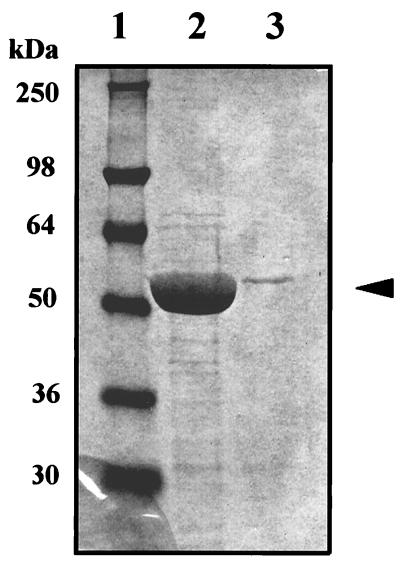
Figure 3.
Purification of SPS2-N-FLAG and CYS-N-FLAG. Extracts (68 mg of protein) prepared from sf9 cells containing SPS2-N-FLAG or CYS-N-FLAG were fractioned on an anti-FLAG M2 affinity gel column (3 ml bed volume) equilibrated with PBS buffer (pH 7.4). After washing the column with three sequential column volumes of PBS (pH 7.4), the bound FLAG fusion proteins were eluted with 6 × 1-ml aliquots of 0.1 M glycine-HCl (pH 3.0). The fractions were neutralized immediately with 1 M Tris·HCl (pH 8.0). The enzymes in each fraction were detected by immunoblotting with anti-FLAG M2 antibody. After combining fractions containing the protein, comparable aliquots of each preparation were analyzed by 12% SDS/PAGE. The relative amounts of enzyme stained with Coomassie blue in the two extracts are shown. Lane 1, molecular weight markers; lane 2, CYS-N-FLAG; lane 3, SPS2-N-FLAG.